Good Manufacturing Practices (GMP) are a set of performance-based requirements and guidelines. Each manufacturer must set up and follow their own quality control processes to guarantee that manufactured products are safe for human consumption. GMPs primarily apply to food and beverage, cosmetics, pharmaceutical products, dietary supplements, and medical device industries. It covers all aspects of production from raw materials, premises, and equipment to the training and personal hygiene of staff. The Food and Drug Administration in the US, the European Medicines Agency, etc., ensure the enforcement of country-specific GMP rules and regulations. Traditional, paper-based, and manual systems not only make it challenging to follow GMPs but also to perform audits. GMP software can address these challenges and simplify the process.
This article will list the top 5 GMP compliance software, including their key features and benefits, and tips to choose the right solution.
What is GMP Software?
GMP software is designed to help manufacturing businesses comply with Good Manufacturing Practices (GMP) by automating, managing, and simplifying quality control, documentation, GMP audits, and regulatory processes, especially in pharmaceuticals, food, and related industries.
GMP apps offer centralized platforms for tracking, managing, and documenting processes required for GMP compliance, such as manufacturing audits, corrective actions, training, and risk assessment. They help ensure product safety, data integrity, and regulatory adherence by automating routine tasks, reducing manual errors, and offering real-time oversight.
Top 5 GMP Software in 2026
Here are the top 5 software that can help you implement GMP in your manufacturing operations.
1. GoAudits GMP Compliance Software
GoAudits GMP audit software supports businesses in maintaining and achieving GMP compliance. With its user-friendly interface and extensive feature set, GoAudits aims to simplify the auditing process, streamline workflows, enhance analytics, and improve report generation capabilities. It is tailor-made for businesses looking to elevate their quality management systems, ensuring that they meet the high standards required in their respective industries.
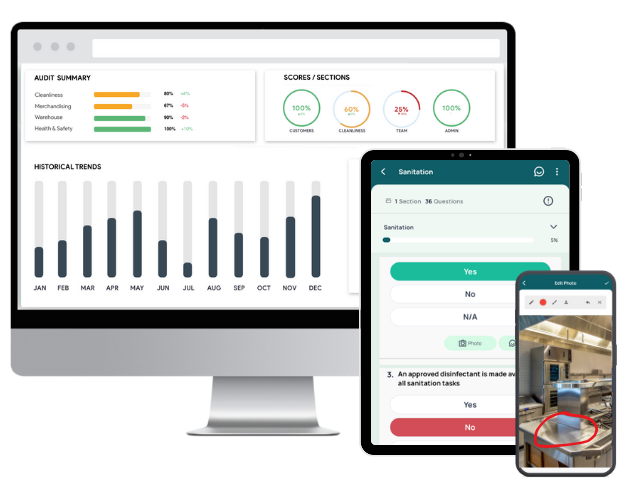
Key features and highlights of GoAudits:
- Move from paper-based audits to digital, using customizable digital checklists that can include photos, signatures, and annotations for more thorough inspections.
- Enables on-the-spot data capture, including evidence through photos, which significantly reduces the chances of errors and omissions.
- Automated generation of detailed audit reports immediately after completion, allowing for quick analysis and decision-making.
- Identifies issues during audits, assigns corrective actions, and tracks them to resolution, ensuring continuous improvement and enhancing operational efficiency.
- View trend analysis over time to track performance improvements or declines.
- Use advanced analytics tools to derive actionable insights from audit data, helping to identify trends, risks, and opportunities.
- Enables benchmarking against standards and past performance to gauge improvements or identify areas needing attention.
Nissin Foods, the manufacturer of Cup Noodles, used the GoAudits inspection app to manage quality control, product and packaging integrity checks, facility inspections, standard operating procedures, and more. What worked for them was GoAudits’ user-friendliness and simple interface. It made it very easy to use for production staff, and the audit completion rates significantly improved after the introduction of the app.
👉 Read our customers’ success stories.
We are now averaging over 96% completion ratios for our GMP audits. With the ability to immediately email the report to the relevant parties, we get real-time information that allows our supervisors to sign off corrective actions and resolve any issues much quicker than before.
Eddie Odie, Quality Control Manager, Nissin Foods
2. InstantGMP
InstantGMP provides an all-in-one platform for managing manufacturing processes, inventory, and quality assurance. It is recognized for its user-friendly design and affordability, making it an ideal choice for small to mid-size enterprises seeking to streamline their GMP compliance processes.
Key features of InstantGMP include the following:
- Streamline creation, production, and tracking of master batch records (MBR) and batch production records (BPR).
- Manage deviations, investigations, and verification of raw materials and finished products.
- Trace, monitor, and record inventory from raw material ordering to final production.
- Store, review, update, and approve production files, records, and videos securely.
- Manage employee training and certifications to maintain GMP compliance.
3. Orcanos
Orcanos offers a semi-all-in-one platform designed to centralize design control, manufacturing, and regulatory compliance into one system. This GMP software caters to those who require tailored solutions for their unique manufacturing processes. Orcanos supports a web, iOS, and Android platform, making it accessible across different devices.
Key features of Orcanos are given below:
- Organize all documents to support compliance and quality management.
- Manage process or system changes in a controlled and efficient way.
- Identify, assess, and mitigate risks in manufacturing and design processes.
- Track and manage employee training to align with the latest GMP requirements.
- Simplify audit planning, execution, and reporting for internal and external audits.
- Support CAPA for non-conformities or potential issues.
4. MasterControl
MasterControl GMP compliance software particularly focuses on current good manufacturing practices (cGMP). It is designed to streamline quality management processes, ensuring products meet the strict regulatory standards set by authorities like the FDA. It offers a suite of tools to manage documents, audits, training, and quality processes comprehensively.
Key features of MasterControl include the following:
- Centralize document management with secure access, version control, and audit trails.
- Risk assessment, mitigation tools, and comprehensive audit management.
- Automate employee training scheduling and tracking for GMP compliance.
- Electronic device history record (DHR) system for production traceability and quality assurance.
- Integrated corrective action management to address non-conformities and prevent recurrence.
5. Tulip
Tulip GMP auditing software enables businesses to streamline work instructions, enhance production visibility, implement efficient machine monitoring, and foster operator training. It promotes lean manufacturing principles, improves quality management, and facilitates compliance audits, ensuring adherence to GMP standards. It is accessible across the web, iOS, and Android devices.
Key features of Tulip include:
- Simplify SOP creation, dissemination, and accessibility.
- Gain real-time insights into production processes for informed decisions.
- Integrate with machinery and IoT devices for automated data collection and monitoring.
- Enable seamless data exchange with other software systems and protect sensitive information.
- Ensure compliance with regulatory standards through digital documentation and e-signatures.
Free GMP Audit Checklists and Templates
You can use one of our GMP audit checklists below to get started. You can also digitize your GMP SOPs into actionable checklists.
- GMP Self-Inspection Checklist
- GMP (Good Manufacturing Practice) Audit
- GMP Inspection Checklist
- GMP Checklist for Breweries
How GMP Compliance Software Helps Implement the 10 GMP Principles
GMP guidelines can be summarized into 10 principles, and GMP compliance software can help you achieve regulatory compliance in each of these steps.

Creation and Implementation of SOPs
Every process potentially impacting product quality must have detailed, written procedures. For example, Standard Operating Procedures (SOPs) establish consistent operational guidelines. They act as a comprehensive ‘instruction manual’ for daily operations, ensuring that every process is performed uniformly.
GMP software simplifies the management of SOPs by digitizing documentation and ensuring easy access and adherence to the most current procedures, promoting consistency and compliance.
Design and Maintenance of Facilities and Equipment
Facilities and equipment should be designed, maintained, and cleaned to safeguard product quality. Regular validation and calibration of equipment are vital to ensure consistent and reliable operations. This requires regular inspections for cleaning and housekeeping standards, as well as equipment checks and equipment cleaning, which can be made easier with an inspection app.
With features for scheduling regular inspections, GMP audit software improves the efficiency and reliability of equipment and facilities maintenance, ensuring they are always in compliance with quality standards.
Quality Assurance in Materials
All materials used in the manufacturing process, including ingredients and packaging, must be of tested quality, clearly identified, and traceable. This ensures the integrity and safety of the final product.
GMP manufacturing software enables better traceability and management of materials, ensuring all ingredients and packaging used in manufacturing are of tested quality and fully compliant.
Controlled and Validated Production Processes
Manufacturing processes need to be clearly defined, controlled, and validated. Quality assurance needs to be embedded in the production process to guarantee consistent quality at every step, every day. Validation of critical processes is essential.
GMP manufacturing software ensures that manufacturing processes are not only defined and controlled but also consistently meet the set quality standards through real-time monitoring and validation.
Rigorous Quality Control
Quality control is paramount, involving testing products at various production stages. These tests ensure the identity, purity, potency, and overall quality of the product, adhering to stringent standards.
Implementing digital quality checks through GMP software enables more efficient and accurate quality control, fitting quality assurance directly into daily operations.
Comprehensive Documentation
Accurate and comprehensive documentation, including records and raw data related to production and distribution, is essential. These documents must be retained for review, showcasing transparency and traceability.
GMP apps enable document management, ensuring that records are always accurate, up-to-date, and easily accessible for audits, enhancing transparency and traceability.
Trained and Qualified Personnel
The role of qualified, trained personnel in quality processes cannot be overstated. Each individual involved in the manufacturing process must possess the necessary education, training, and experience to perform their tasks effectively. GMP software gives quick access to SOPs and digital checklists on a mobile device, making it easier to train staff.
Validation and Control of Changes
Any changes to the manufacturing process must undergo thorough review, validation, and documentation. This ensures that the quality of the product remains uncompromised by any modifications.
GMP compliance software streamlines the change management process, ensuring that any modifications in the manufacturing process are thoroughly reviewed, validated, and documented without compromising product quality.
Handling of Complaints and Recalls
Effective systems for managing complaints and product recalls are crucial. This involves the investigation and review of complaints and implementing appropriate corrective actions, ensuring ongoing product safety and quality.
Through efficient tracking of corrective actions and complaints, GMP software ensures that all issues are addressed promptly and effectively, maintaining product safety and quality.
Regular Auditing for Compliance
Conducting regular audits is key to ensuring adherence to GMP guidelines. These audits help identify areas for improvement and ensure the implementation of corrective actions, maintaining the highest standards of quality.
GMP audit software optimizes audit processes by providing tools for planning, execution, and documentation, ensuring regular compliance checks are conducted efficiently and effectively.
How to Choose the Best GMP Software: Features & Factors to Consider
Here are some points you should consider to choose the right GMP software:
Compare the Cost and Budget
The first step in selecting the right GMP software is to evaluate the cost against your budget. GMP software varies widely in price, depending on its features, scalability, and customization options. It’s important to consider not only the upfront costs but also long-term expenses, including maintenance, updates, and support. Aim for a GMP app that offers the best value for money, balancing cost with the features and reliability your business needs. Additionally, exploring vendor incentives or discounts for larger projects can offer savings.
Look for Essential Features
The ideal GMP software should offer a comprehensive suite of features tailored to the specific needs of your industry and operational requirements. Essential features to look for include the following:
- CAPA Management: Essential for addressing and preventing quality issues, ensuring continuous compliance and quality improvement.
- Paperless Documentation: Supports paperless documentation and facilitates efficient management and retrieval of records, offering benefits like improved accessibility, reliability, and security of records.
- Real-time Analytics: Provides instant access to data for timely decision-making, with comprehensive reporting tools for continuous performance monitoring.
- Mobile App for Audits: Offers a convenient tool for conducting audits on the go (even without internet access), ideal for multi-location operations or frequent inspections.
- Custom Checklists: Allows for tailored GMP audits and inspections, focusing on specific operational needs or regulatory standards for thorough compliance.
Read Customer Reviews and Testimonials
Customer feedback can provide invaluable insights into the GMP software’s performance, reliability, and user satisfaction. Reviews and testimonials offer a glimpse into other users’ experiences, highlighting the GMP compliance software’s strengths and potential limitations. It can help gauge whether a particular GMP software meets your expectations and industry standards.
Check Whether It Offers Free Trials
Free trials are an excellent way to evaluate a GMP audit software’s functionality, user-friendliness, and fit for your business before making a financial commitment. Some software providers offer free trials for a week or two, allowing you to test features and assess the GMP compliance software’s impact on your operations.
Look for Security Measures and Compliance
Security is paramount in GMP software, given the sensitive nature of the data involved. Ensure the GMP compliance software provides encryption protocols and access control settings, and complies with industry standards like GDPR or HIPAA. This will protect your business and customer data from potential threats and ensure regulatory compliance.
Analyze Scalability Features
Scalability is crucial as your business grows. The GMP software should be able to accommodate increasing data volumes, user roles, and operational complexity without significant disruptions. Assess the GMP app’s ability to scale and the provider’s support for upgrades and expansions.
Consider the Time Required to Learn and Use It
The ease of use and learning curve of GMP software is essential for smooth adoption and utilization. Look for intuitive interfaces, comprehensive training resources, and strong customer support to ensure a smooth transition and ongoing usability.






