Running a laboratory without regular audits or inspections quickly creates blind spots: undocumented changes to methods, overdue calibrations, or safety issues that only surface when something goes wrong. Laboratory audits and inspections make these risks visible by checking how your people, processes, and records perform against regulatory and accreditation requirements.
In this article, you’ll learn the main types of laboratory audits, how often they should be carried out, the steps to perform them, and how they underpin a lab quality management system (QMS). You’ll also see how laboratory audit software like GoAudits and digital checklists prepare you for internal and external assessments at any time.
- What are Laboratory Inspections?
- Lab Audits vs Lab Inspections
- Types of Laboratory Audits & Inspections
- Steps to Conduct Internal Laboratory Inspections & Audits
- Perform Laboratory Audits with Laboratory Audit Software
- Key Factors to Consider When Choosing Laboratory Compliance Software
- Lab Quality Management System: An Overview
- Steps to Implement a Lab Quality Management System
- How Laboratory Inspections Sustain Lab Quality Management System
What are Laboratory Inspections?
A laboratory inspection or laboratory audit is a systematic review of your lab’s facilities, processes, records, and staff competence to verify compliance with internal policies and external standards such as ISO 15189, ISO/IEC 17025, and GLP.
All laboratories involved in testing, calibration, or research that generate data used for regulatory submissions, public health decisions, or product quality assurance require audits to demonstrate reliability and integrity. This includes clinical, pharmaceutical, environmental, food, and industrial labs.
Common laboratory audit findings
Frequent nonconformities identified during audits include:
- Documentation: Incomplete or inconsistent records, missing revisions, poor traceability.
- SOPs and methods: Outdated or unapproved procedures; methods not followed as written.
- Personnel: Missing or outdated competency assessments and training records.
- Equipment: Overdue calibration, missing maintenance logs, or unverified performance checks.
- Sample and data management: Labelling errors, chain-of-custody gaps, or unprotected data changes.
- Quality control: Weak internal QC, poor follow-up on EQA/proficiency testing failures.
When and How Often Lab Audits Are Performed
Audits are conducted by qualified professionals depending on the audit type:
- Quality assurance (QA) managers perform internal audits to ensure daily operations comply with established quality systems.
- External assessors conduct independent evaluations to identify gaps that internal teams may overlook.
- Accreditation bodies, such as ISO or national regulatory authorities, perform formal assessments required for accreditation or certification.
Audit frequency depends on the laboratory’s activities, regulatory requirements, and risk level. Internal audits are typically carried out annually or semi-annually. External audits or accreditation assessments usually occur every one to two years, though follow-up or surveillance audits may be scheduled more frequently if significant issues arise.
Why are Laboratory Audits Performed?
Lab audits are performed for several reasons, including:
- Confirms that laboratory activities align with established protocols and international standards such as ISO 9001, ISO/IEC 17025, or ISO 15189 to maintain reliability and compliance.
- Evaluates whether personnel possess the required skills and knowledge, ensuring that training programs effectively support accurate and consistent laboratory performance.
- Reviews testing methods, equipment calibration, and documentation to confirm that results are precise, reproducible, and scientifically valid.
- Detects process weaknesses or nonconformities, enabling timely implementation of measures to prevent recurrence and enhance operational efficiency.
- Promotes regular performance reviews and process optimization, establishing a culture of quality, accountability, and sustained improvement across all laboratory functions.
Lab Audits vs Lab Inspections
In simple terms, a laboratory inspection is a shorter, often checklist-based review of day-to-day conditions and safety, while a laboratory audit is a deeper, system-wide evaluation of your quality management system and data integrity.
| Aspect | Lab Inspection | Lab Audit |
| Scope | Snapshot assessment focused on compliance, safety, and operations at a specific point in time | Comprehensive evaluation of all laboratory systems, documentation, and processes |
| Objective | Identify visible risks, unsafe practices, or regulatory non-compliance | Assess the effectiveness of quality management systems and ensure regulatory conformity |
| Approach | Targeted, routine, and often checklist-based | Systematic, documented, and process-oriented |
| Purpose | Confirm day-to-day policy adherence and detect immediate hazards | Evaluate system robustness and data integrity for sustainable quality improvement |
| Outcome | Findings requiring prompt correction | Detailed report recommending corrective and preventive actions |
| Conducted By | Laboratory management, safety officers, or external regulators | Internal quality team, accreditation bodies, or independent auditors |
| Types & Frequency | Routine or unannounced; performed daily, weekly, or after incidents | Scheduled and planned; internal audits are typically annual, external audits follow multi-year cycles |
| Standards Referenced | Regulatory and safety guidelines | ISO 15189, ISO 17025, CLIA, GLP, GMP, and other compliance frameworks |
| Focus Areas | Equipment condition, safety practices, and facility upkeep | Documentation accuracy, personnel competency, procedural adherence, and system performance |
| Typical Duration | Short-term, often completed within hours | Long-term, requiring a detailed review over days or weeks |
Types of Laboratory Audits & Inspections
The main types include internal audits, external laboratory audits, and laboratory safety inspections.
Internal Laboratory Audits
Internal audits are conducted by personnel within the organization to evaluate how well the laboratory adheres to its quality management system. They focus on verifying compliance with internal procedures, ISO standards, and operational requirements.
During an internal audit, auditors review documentation, observe laboratory practices, and check equipment calibration and maintenance records. The goal is to detect inconsistencies, non-conformities, or procedural weaknesses before they escalate. Internal audits also ensure staff accountability and reinforce best practices.
External Laboratory Audits
External lab audits are performed by independent bodies, accreditation agencies, or regulatory authorities. They provide an objective assessment of the laboratory’s competence, data integrity, and compliance with industry standards.
External auditors examine documentation, test records, personnel qualifications, and quality control measures. They also assess the traceability of measurements and the validity of test results. Passing an external audit strengthens a laboratory’s credibility and demonstrates its commitment to consistent, high-quality performance.
Laboratory Safety Inspections
These focus on evaluating workplace conditions, equipment safety, and compliance with health and safety regulations. Inspectors look for potential hazards such as chemical spills, improper waste disposal, or malfunctioning safety equipment.
These inspections ensure that emergency protocols, personal protective equipment (PPE), and safety signage are in place and effectively used. Regular safety inspections help prevent accidents, reduce risks, and foster a culture of responsibility within the laboratory environment.
Other types of lab audits include:
| Audit Type | Description | Performed By |
| Regulatory Audits | Official inspections to ensure adherence to legal and regulatory requirements | Government/regulators |
| Certification Audits | Audits for accreditation based on international standards | Accrediting body |
| Supplier Audits | Evaluation of labs as suppliers by clients or third parties | Client or third-party |
| For-Cause Audits | Triggered by specific quality or compliance issues | Client/regulator |
| Surveillance Audits | Scheduled audits between certification cycles to ensure ongoing compliance | Accrediting body |
| Horizontal Audits | Cross-departmental process evaluations | Internal or external auditors |
| Vertical Audits | Focused audits on specific departments or functions | Internal or external auditors |
| Test Audits | Sampling audits to evaluate effectiveness and compliance, or a data reliability check | Internal or external auditors |
Steps to Conduct Internal Laboratory Inspections & Audits
Follow these steps to perform a thorough lab inspection.
1. Define Scope and Objectives
Begin by setting clear goals for the inspection. Determine which laboratory equipment, processes, and safety practices will be reviewed. Establish measurable objectives, such as assessing compliance with internal protocols, verifying calibration records, or evaluating sample handling procedures. A well-defined scope ensures that the inspection remains focused and productive.
2. Assemble an Inspection Team
Select qualified personnel with technical expertise and an understanding of laboratory operations. Include members from different departments to enhance the accuracy and fairness of the inspection and ensure a balanced perspective. Assign specific responsibilities to each team member, such as documentation review, equipment evaluation, or safety assessment.
3. Use Digital Checklists to Perform Lab Inspections
Use digital checklists to streamline the process and ensure consistency. Digital tools allow real-time data entry, photo documentation, and automated scoring. They also reduce paperwork and help track compliance trends over time. Ensure that each checklist aligns with relevant standards to maintain uniformity.
You can use the free checklists GoAudits offers to perform lab audits up to 5 times faster:
- Laboratory Equipment Checklist
- Laboratory Checklist Template
- ISO 15189:2022 Checklist
- ISO 17025 Checklist
- ISO 9001 Checklist
- ISO 9001 Audit Checklist
4. Identify and Classify Findings
Record all observations and classify them according to their severity: minor, major, or critical. Distinguish between non-conformities, observations, and opportunities for improvement. Use objective evidence to support each finding. This allows for clear prioritization and helps in addressing high-risk issues promptly.
5. Implement Corrective Actions and Monitor Their Impact
Develop corrective action plans for each finding, assigning clear responsibilities and timelines. Implement the actions systematically and document every step to reinforce accountability and improve laboratory performance. After completion, monitor the effectiveness of these actions to ensure that the underlying issues are resolved and do not recur.
6. Prepare and Share the Report with Relevant Stakeholders
Compile a comprehensive report summarizing the inspection process, key findings, and corrective measures. Include recommendations for preventive actions and areas requiring further attention. Share the report with management, quality assurance teams, and other stakeholders. Encourage feedback to enhance transparency and promote continuous improvement within the laboratory.
Perform Laboratory Audits with Laboratory Audit Software
During an external audit, assessors expect to see not only SOPs and policies but also evidence that internal audits, safety inspections, and CAPAs are performed consistently and on time. Laboratory audit software like GoAudits makes this easy to demonstrate with a few clicks instead of hunting through paper folders and spreadsheets.
You can easily perform detailed inspections using custom checklists, attach visual evidence, and automatically generate professional, branded reports, all from one platform. With real-time insights, historical data, and corrective action tracking, you gain full visibility into performance, trends, and compliance status, ensuring that every audit drives operational excellence.
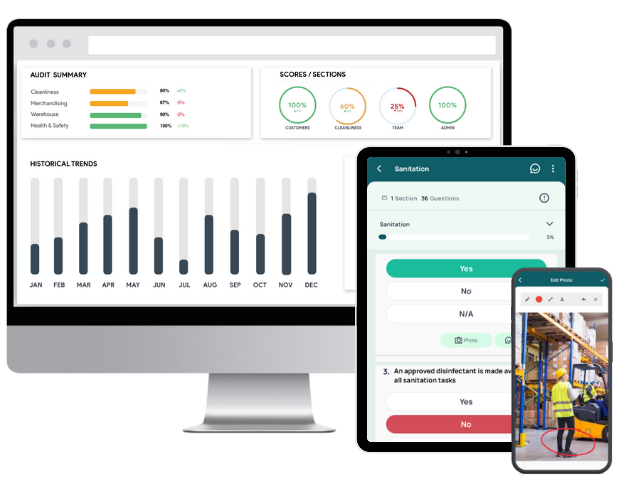
With GoAudits, you can:
- Standardize internal audits & inspections: Use configurable digital checklists for internal audits, safety rounds, and housekeeping checks. Align them with ISO 15189, ISO/IEC 17025, GLP, or your own SOPs, and ensure every auditor follows the same structure.
- Capture clear evidence during every audit: Perform audits on mobile devices, even offline. Add photos, comments, e-signatures, timestamps, and geolocation so findings are fully documented and traceable.
- Generate audit-ready reports instantly: Turn completed audits into branded PDF or Excel reports with one click. Share them with management or accreditation assessors without manual formatting or copy-paste.
- Track CAPA from finding to closure: Assign corrective actions directly from findings, set deadlines and owners, and monitor status in real time. See at a glance what’s overdue, what’s recurring, and where risk is building.
- Automate reminders and workflows: Configure alerts for missed inspections, high-risk findings, or overdue CAPAs. Route reports to the right managers automatically, so nothing falls through the cracks.
- Monitor performance across sites and teams: Use dashboards to track completion rates, recurring issues, and trends by area (e.g., sample handling, equipment, documentation), with a clear view of where to focus training or process improvement.
Key Factors to Consider When Choosing Laboratory Compliance Software
Selecting the right laboratory compliance software requires careful evaluation of factors that directly impact accuracy, efficiency, and regulatory adherence. Let’s explore some of the most important of these factors.
Unique Laboratory Requirements
Every laboratory operates under specific regulatory frameworks and workflows. The software you choose should align with your lab’s unique standards, whether you handle clinical testing, pharmaceuticals, food safety, or research. It must support your documentation style, data management practices, and reporting needs. Look for solutions that allow flexible configuration and integration with existing instruments and systems.
Budget Friendliness
Cost-effectiveness plays a significant role in any software investment. While it’s important to manage expenses, avoid choosing solely based on price. Instead, evaluate the total cost of ownership, including setup, maintenance, updates, and scalability. A slightly higher upfront cost may lead to better long-term value if the software reduces compliance risks and operational delays.
Essential Features
Modern compliance software should include tools that simplify and automate quality management. Key features to look for include:
- Digital checklists to standardize procedures and reduce manual errors
- Mobile auditing to perform inspections and audits anytime, anywhere
- Reporting and analytics to track performance metrics and identify compliance gaps
- CAPA management to document and monitor corrective and preventive actions effectively
- Workflow automation to ensure consistency and speed in recurring tasks.
These features collectively help maintain data integrity, meet regulatory expectations, and improve laboratory productivity.
Intuitiveness and User-Friendliness
Ease of use determines how quickly your team adapts to the new system. Choose software with an intuitive interface that minimizes the need for extensive training. A clean dashboard, logical navigation, and clear instructions allow users to perform tasks efficiently without constant technical support.
Positive Customer Reviews and Testimonials
Real user experiences provide valuable insights into software performance. Read verified reviews and testimonials to assess reliability, user satisfaction, and post-implementation support. Consistently positive feedback from laboratories of similar size or industry is a strong indicator of dependable functionality.
Round-the-Clock Customer Support
Continuous support ensures uninterrupted operations. Select a provider offering 24/7 assistance through multiple channels such as chat, email, and phone. Quick issue resolution, proactive communication, and accessible technical guidance are essential to maintaining compliance and minimizing downtime.
Lab Quality Management System: An Overview
A laboratory quality management system (QMS) is a structured framework that ensures laboratory processes, operations, and outputs consistently meet defined quality standards. It covers every stage of laboratory activity, from sample collection and testing to result reporting and continuous improvement.
A well-defined laboratory QMS integrates quality assurance, quality control, document control, risk management, and continual improvement practices. It provides clear procedures for handling test methods, equipment calibration, data integrity, and personnel competence.
The three most important frameworks for a laboratory quality management system are:
- ISO 15189
- ISO/IEC 17025
- Good Laboratory Practices (GLP)
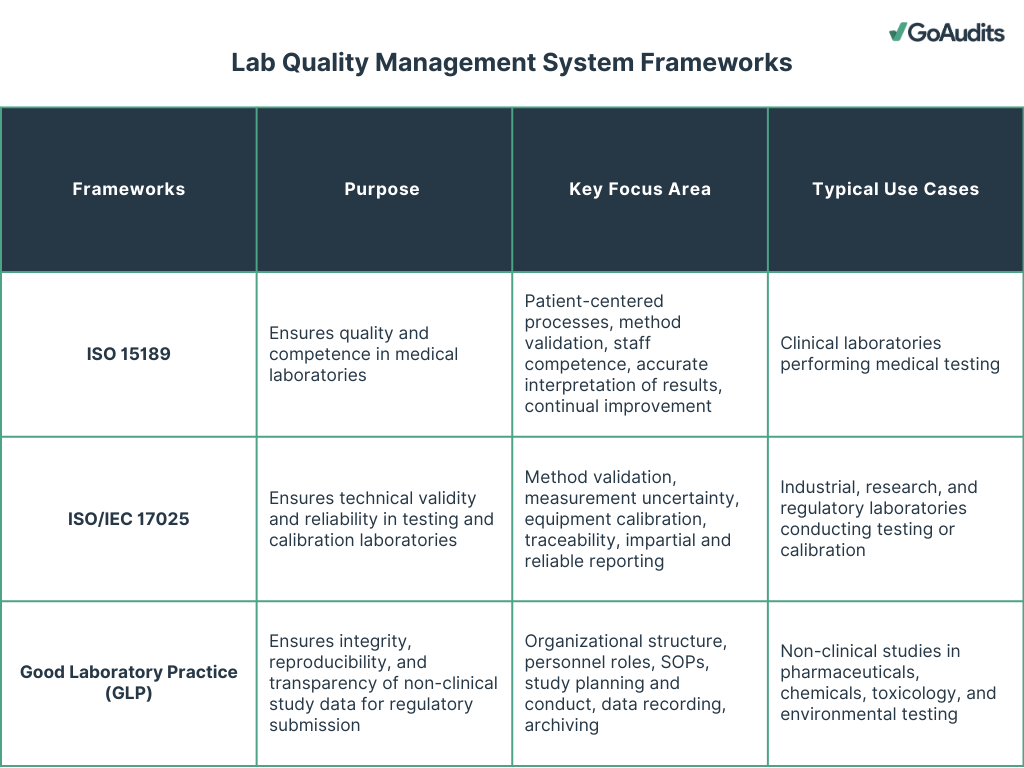
ISO 15189, ISO 17025, and GLP share a common foundation: ensuring quality, consistency, and credibility in laboratory operations. They help you manage risks, streamline processes, and achieve accreditation that validates your laboratory’s technical competence and data reliability.
Importance of Lab Quality Management System
Here are some reasons why it’s important:
- Establishes uniform protocols and record-keeping methods, ensuring consistency, traceability, and clarity across all laboratory operations.
- Implements systematic quality checks that minimize analytical errors and strengthen the validity of diagnostic results.
- Ensures that test results are precise and reproducible, supporting timely and effective clinical decisions for better patient care.
- Aligns laboratory practices with recognized global standards, simplifying audits and accreditation by regulatory and certifying bodies.
- Identifies potential process gaps early, enabling proactive corrective actions that lower the likelihood of costly mistakes and rework.
- Encourages staff ownership of quality objectives while fostering ongoing training and performance improvement throughout the laboratory.
Steps to Implement a Lab Quality Management System
Follow the steps below to implement a lab QMS:
1. Establish Leadership Commitment and Define Quality Policy and Objectives
Begin by securing commitment from top management. Leadership must endorse quality as a core organizational value and allocate the necessary resources. Define a clear quality policy that reflects the laboratory’s mission, scope, and compliance obligations. Establish measurable quality objectives aligned with customer needs and regulatory standards.
2. Conduct a Gap Analysis and Develop an Implementation Plan
Evaluate the current system against applicable standards, and identify existing strengths and areas for improvement. Based on this assessment, create a detailed implementation plan outlining specific actions, timelines, responsibilities, and required resources. The plan should prioritize critical gaps that affect quality or compliance.
3. Establish the Quality Management Structure and Assign Responsibilities
Define an organizational structure that supports quality management functions. Appoint a quality manager or designate a quality assurance officer responsible for overseeing QMS activities. Clearly define roles, responsibilities, and reporting lines to ensure accountability across all operational levels.
4. Develop, Control, and Implement Documentation and Standard Operating Procedures (SOPs)
Create a comprehensive documentation system that includes policies, procedures, work instructions, and forms. Develop standard operating procedures for all critical processes, ensuring clarity, consistency, and traceability. Implement document control measures to manage revisions, approvals, and accessibility to prevent errors caused by outdated information.
5. Implement Quality System Essentials (QSEs)
Introduce the fundamental elements of the QMS, such as personnel training, equipment management, process control, data management, customer service, and occurrence management. Each QSE must be integrated into daily operations to maintain uniform practices and promote continual quality improvement.
6. Apply Internal and External Quality Controls and Assessments
Establish internal quality controls to monitor the accuracy and precision of testing processes. Participate in external quality assessment programs to compare performance with peer laboratories. Analyze results systematically to identify trends, deviations, and improvement opportunities.
7. Conduct Internal Audits and Management Reviews
Plan and conduct internal audits to evaluate compliance with established procedures and identify nonconformities. Present audit findings to management for review. During management reviews, assess overall QMS performance, resource needs, customer feedback, and opportunities for enhancement.
8. Implement Corrective Actions and Prepare for Accreditation
Address identified nonconformities through well-documented corrective and preventive actions. Verify the effectiveness of implemented solutions. Once all system components are in place and functioning as intended, prepare for external accreditation. Ensure readiness by conducting a pre-assessment and resolving any remaining deficiencies.
How Laboratory Inspections Sustain Lab Quality Management System
Laboratory inspections are integral to sustaining an effective QMS. They provide a structured approach to verify that laboratory operations meet established quality benchmarks and adhere to international standards. Lab audits ensure that every process, from sample handling to data reporting, aligns with defined regulatory and quality requirements.
Regular inspections detect potential risks, deficiencies, and non-conformities that could compromise test accuracy or reliability. By identifying these gaps early, laboratories can implement corrective and preventive actions that enhance operational performance and minimize future errors. This mitigates risks and ensures continuous improvement across all functional areas.
Lab audits also validate the consistency between documented policies and actual laboratory practices. They assess whether staff follow SOPs and whether management systems function as intended in real-world conditions. This strengthens process integrity and ensures that compliance is not limited to paper-based documentation.
Moreover, inspection outcomes reinforce accountability at every organizational level. When personnel understand that quality is continually monitored and evaluated, adherence to best practices becomes part of the laboratory’s culture. This fosters transparency and encourages responsible behavior in maintaining quality standards.
Consistent inspections also enhance stakeholder confidence and public credibility. Clients, regulatory authorities, and accreditation bodies gain assurance that the laboratory operates with precision, reliability, and integrity. Such trust is essential for maintaining long-term professional relationships.
Laboratory inspections support sustainable quality improvement initiatives. They provide data-driven insights that guide strategic planning, optimize resource use, and strengthen the overall QMS. Making inspections a continual process rather than a one-time activity helps laboratories ensure lasting compliance, performance excellence, and consistent delivery of accurate results.





