Pick your industry, then choose an inspection checklist template:
★★★★★ 5/5
“Highly recommended!”
GoAudits has assisted us to move dramatically through our quality control scores, internal and external. Any recommendation or request we made was immediately actioned upon.

★★★★★ 5/5
“96% completion on GMP audits”
With instantly compiled and emailed reports, we get real-time data that allows our supervisors to sign off corrective actions and resolve issues much quicker.


★★★★★ 5/5
"Full picture of store performance”
The GoAudits team was super engaged and adapted the platform to suit our needs. We were able to roll out to all the area managers with ease.


Frequently Asked Questions
Are your audit checklist templates customizable?
Absolutely! Our audit checklist templates are fully customizable using our drag-and-drop form builder tool. Modify existing templates by adding or removing items, adjusting categories, and personalizing the layout. All you need to do is sign up for our Free Trial to see it in action.
Alternatively, you can share your existing checklists and our team will digitize them for you inside our inspection app.
What is an audit checklist template?
An audit / inspection checklist template is a structured tool used to guide the audit or inspection process. It outlines key items, tasks, or criteria that need to be evaluated, ensuring a comprehensive assessment.
These templates help streamline audits by providing a consistent approach for collecting and analyzing data, making it easier to identify compliance issues, track progress, and improve overall performance.
How can I use an inspection checklist template?
You can use our library of inspection checklist templates by following these steps:
- Select the template relevant to your needs.
- Review the checklist items to ensure they cover everything you need to inspect.
- Customize the checklist to fit your specific requirements, adding or removing items as necessary.
- Use the GoAudits inspection app to easily manage your checklist and keep all your data in one place.
- Conduct your inspection, checking off each item as you go, adding photos and comments.
- Found an issue? Assign a corrective action to the relevant team member.
- Generate an instant report, share with colleagues and identify areas for improvement.
What are the benefits of using an audit checklist sample?
Using an inspection checklist sample serves as a helpful starting point, saving you time and effort compared to creating a checklist from scratch. These samples cover essential items and best practices relevant to various industries, allowing you to trust that you’re addressing important aspects. You can also easily customize the sample to fit your specific needs, making it a practical and efficient tool for your auditing process!
Can I download your inspection checklist samples in PDF?
Yes, you can download our inspection checklist templates in PDF format. However, we highly recommend using them digitally within the GoAudits app. By doing so, you can easily access, edit, and update your checklists on the go. The app also allows you to collect data in real-time, generate instant reports, and store all your inspection information in one convenient place, making your auditing process much more efficient!
How can I improve my inspection processes using checklist templates?
You can definitely make your inspection processes more effective by using checklist templates. Here are a few steps you can take:
Standardize Procedures: Use checklist templates to create a consistent approach to operations, ensuring that all team members follow the same process.
Tailor to Your Needs: Customize the templates to include specific items relevant to your business, helping you focus on critical areas and compliance requirements.
Streamline Data Collection: Utilize digital checklist templates within the GoAudits app to capture data in real-time, reducing errors and saving time on data entry.
Analyze Results: After completing inspections, generate instant reports and view data in handy dashboards to identify trends, recurring issues, and areas for improvement.
Facilitate Training: Use the templates as training tools for new team members, helping them understand what to look for during inspections.
By leveraging checklist templates in these ways, you can enhance efficiency, accuracy, and overall effectiveness of your operations!
How to create inspection reports based on templates?
Creating inspection reports based on templates is easy with GoAudits! Here’s how you can do it:
Use Our Templates: Start by selecting a checklist template that suits your inspection needs. You can customize it as needed before your inspection.
Conduct the Inspection: As you go through the checklist on-site, you can input data directly into the app. This real-time data collection ensures accuracy and saves you time.
Instant Reporting: One of the standout features of GoAudits is our instant reporting capability. Once your inspection is complete, you can generate detailed reports with just a few taps. This eliminates the need to write reports back at the office, saving you valuable time and allowing you to focus on other tasks.
Access and Share: The generated reports can be easily accessed within the app, and you can share them with your team or stakeholders right away.
By using GoAudits, you streamline the entire inspection and reporting process, making it faster, more efficient, and hassle-free!
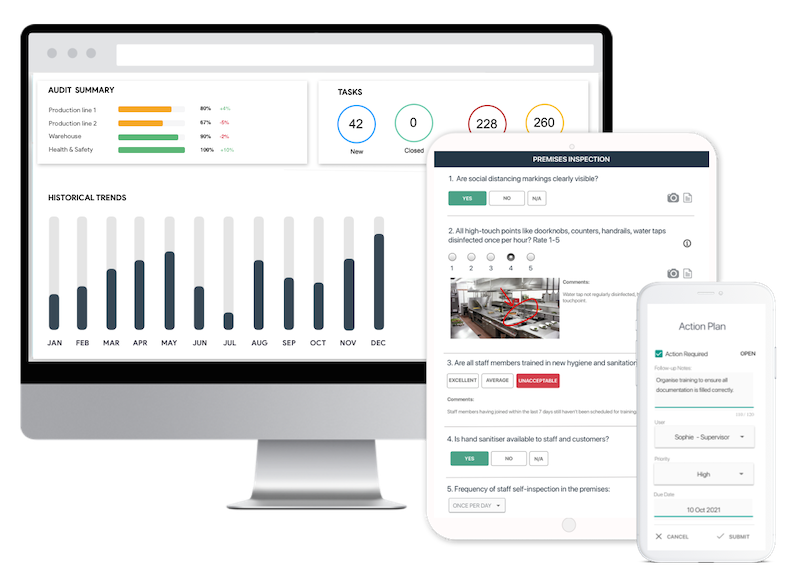
Easy Start with Digital Checklists
- Capture photos as proof of compliance or areas needing attention
- Instantly generate and share detailed reports after the inspections
- Assign follow-up tasks and track completion on a centralized dashboard