GMP Audit Checklist & Templates
Template Library > Food & Hospitality Checklists > GMP Audit Checklist
Conduct Faster Internal Audits with GMP Audit Checklists
Good Manufacturing Practice (GMP) audit checklists are crucial tools for ensuring compliance with industry standards and enhancing product quality. These checklists provide a structured approach to assessing manufacturing processes, helping businesses identify gaps and areas for improvement. Using a comprehensive GMP audit checklist can help businesses ensure that their operations adhere to regulatory requirements, reducing the risk of non-compliance and maintaining high product standards.
Key elements of this GMP audit checklists include:
- Verify cleanliness, maintenance, and proper functioning of equipment.
- Ensure staff are adequately trained and follow GMP guidelines.
- Check that all records are accurate, complete, and up-to-date.
- Assess procedures for quality testing and control.
- Confirm that safety practices are in place and followed.
With the GoAudits Food Safety Software, you can:
- Eliminate paperwork: conduct efficient digital audits
- Customise this template or easily create your own
- Save time with instant reports & assign corrective actions
Management Commitment
1. Does senior management demonstrate commitment to safe food production and handling through: • Promoting food safety awareness throughout the organization? • Facilitating communication relating to food safety issues and incidents? • Providing adequate resources to fully implement the HACCP system to achieve compliance with the BSI HACCP & GMP Certification Criteria?
|
Photo
Comment
|
Continual Improvement
1. Has the effectiveness and continual improvement of the HACCP system been demonstrated through the review of internal verification activities, non-conforming product actions, corrective actions, and the results of external audits? New scientific developments, advances in technology, and industry best practices should also be considered.
|
Photo
Comment
|
Food Safety Policy
1. Will the organization develop a policy that states the organization’s commitment and measurable objectives for the supply of safe and suitable food products that meet customer expectations and legal requirements in the country of manufacture and the country of sale? Will a program to measure and improve food safety culture be established and maintained?
|
Photo
Comment
|
Roles, Responsibilities And Authorities
Controls For Documented Information
1. Has a system to manage documented information (electronic and hardcopy) been implemented to ensure the currency of documentation in use and provide a system for records to be retained and readily retrieved? Documentation and record keeping should be appropriate to the nature and size of the organization and sufficient to verify that the HACCP controls are in place and being maintained. This may include, but is not limited to: • The responsibilities for the development, maintenance, and authorization of all documentation within the HACCP system • Methods of ensuring obsolete documents are removed from use • Responsibilities for the communication of changes to documentation within the HACCP food safety system • Methods for ensuring the security of the documented HACCP food safety system • The method of destruction and control of customer-owned/branded/trademarked documentation, product, and packaging
|
Photo
Comment
|
Document Register
1. Has a document register (list) of the documents referenced in the HACCP system been developed? This may include, but is not limited to the following: • HACCP team composition • Product description and intended use • Hazard analysis, including risk assessment and associated scientific references • CCP determination • Critical limit validation • HACCP audit table • Specifications (raw materials and finished product) • Formulations (recipes) • Prerequisite programmes • Standard operating procedures and work instructions • Policies • Forms
|
Photo
Comment
|
2. Does the organization have access to, and control of, external documents or references required to maintain the HACCP system? This may include but is not limited to food safety statutory and regulatory requirements, codes of practice, guidelines, and standards appropriate to the country in which the food products are manufactured and sold.
|
Photo
Comment
|
HACCP System
The HACCP Team
Scope And Purpose Of The HACCP Plan
2. Will the purpose of the HACCP system include the intent that all food safety hazards will be identified and controlled? Food safety hazards may include but are not limited to: biological, chemical, physical (foreign matter), allergen, and radiological hazards as appropriate to the products in the scope of the HACCP plan.
|
Photo
Comment
|
Product Description And Intended Use
3. A product description for each product or group of products shall detail the following information: Composition (e.g. formulation/ingredients) Physical and chemical characteristics (e.g. final product aW, pH, addition of preservatives) Production methods and technologies (e.g. heat treatment, high-pressure processing (HPP), freezing, drying, brining, etc.) Primary and secondary packaging (e.g. type of packaging used, durability, functional effect on food safety such as the extension of shelf life, etc.) Storage, handling, and distribution methods (e.g. refrigerated/ambient transport requirements) Shelf life (including best-before or use-by-date coding) Intended use of the product(s) Labeling requirements including any claims as per local legislation in the country of sale Allergens as per local legislation in the country of sale Sensitive consumers Some or all of this information may be contained within finished product specifications.
|
Photo
Comment
|
Process Flow Diagram
1. Is a process flow diagram documented for each product or group of products? Is every step in the process(es) identified and sufficiently detailed to include the sequence and interaction of steps, inputs, outsourced processes, intermediate products, rework, end products, waste, and by-products relevant to the process? Complex manufacturing operations may be broken into a series of linked flow diagrams to provide a clear and accurate representation of the process flow.
|
Photo
Comment
|
Hazard Analysis And Control Measures
1. Will a hazard analysis be undertaken and documented for each step of the process and process inputs as identified in the flow process? Will the HACCP team reference the verified process flow diagram in the hazard analysis to identify all potential food safety hazards (as identified in the purpose of the HACCP plan) which need to be prevented, eliminated, or reduced to accepted levels?
|
Photo
Comment
|
3. Are identification and assessment of hazards ungrouped? (e.g. foreign matter which shall be separated into wood splinters, packaging materials, hair, etc.). Is the identification of potential hazards also taken into consideration of the hazards reported in food recalls and outbreaks of foodborne illness as appropriate to the product, process, and global supply chains?
|
Photo
Comment
|
7. When determining significant hazards, does the HACCP team consider the following as applicable to the product and process? • Hazards known to be associated with the type of food, ingredients used in the product, and process steps • Likelihood of occurrence of hazards, taking into consideration prerequisite programs, in the absence of additional control •Likelihood and severity of adverse health effects associated with the hazards in the food in the absence of control • Identified acceptable levels of the hazards in the food (e.g. permissible additives and maximum residue limits defined by regulations in the country of sale) • The food-handling environment and equipment used to produce the food product • The likelihood of survival and/or growth of pathogenic microorganisms • The potential for the presence of toxins (e.g. mycotoxins), chemicals (e.g. pesticides, drug residues), or foreign matter (e.g. glass, metal, soft plastic) • The intended use and/or probability of the product being mishandled by potential consumers that may cause the food to become unsafe Guidance note: There is no specific methodology required to be used to determine the significance of hazards.
|
Photo
Comment
|
8. For all hazards determined to be significant, is there at least one control measure designed to prevent or eliminate the hazard, or reduce the hazard to an acceptable level? Control measures may reference the application of a pre-requisite program to reduce, prevent or eliminate a significant hazard (e.g. cleaning of equipment to prevent cross-contact of food allergens from one food to another food that does not contain that allergen). In other instances, the control measures shall be applied within the process at critical control points (CCPs).
|
Photo
Comment
|
Critical Control Points
1. Will the HACCP team determine the critical control points for hazards identified in the hazard analysis as significant hazards? Will CCPs be established at steps where control is essential to safe food production and where a deviation could result in potentially unsafe food? There may be more than one CCP in a process in which control is applied to address the same hazard. If no control measures exist at any step for an identified significant hazard, then is the product or pro- cess modified? Guidance note: There is no specific methodology required to be used to determine CCPs.
|
Photo
Comment
|
HACCP Audit Table
1. Will a HACCP audit table be developed, documented, and applied which includes all steps of the process where CCPs have been identified? Will the corresponding monitoring activities, corrective actions in the case of deviations, and verification activities be documented for each CCP?
|
Photo
Comment
|
Validated Critical Limits
2. Are critical limits measurable or observable? There may be multiple critical limits identified for a CCP, (e.g. heat treatments may include critical limits for time at temperature). Are critical limits for control measures at each CCP specified and scientifically validated to obtain evidence that they are capable of controlling hazards to an acceptable level if properly implemented? Guidance note: Critical limit criteria may include minimum and/or maximum values (e.g. temperature, time, pH, available chlorine, contact time, conveyor belt speed, flow rate, etc.).
|
Photo
Comment
|
3. Is validation of critical limits considered if the appropriate critical limit has been determined and the capability of the organization to consistently achieve the limit(s)? Validation data shall be documented. Guidance note: validation data may include, but is not limited to: regulations, industry codes of practice, guidance from competent authorities, studies conducted by equipment manufacturers, and site-specific information confirming their capability to consistently achieve the critical limit(s).
|
Photo
Comment
|
System To Monitor Control Of CCPs
3. Will the monitoring procedures for CCPs be capable of timely detection of a deviation from the critical limit to allow non-conforming products to be isolated? The monitoring of CCPs should be continuous, where possible. If monitoring is not continuous, then is the frequency of monitoring sufficient to ensure that the CCP is under control? (e.g. critical limits based on observation such as the application of the correct label to a product containing allergens, needs a monitoring frequency based on the capability of the organization to prevent the distribution of non-conforming products). Guidance note: physical and chemical measurements are usually preferred to microbiological testing because physical and chemical tests can be done rapidly and can often indicate the control of microbial hazards associated with the product and/or the process.
|
Photo
Comment
|
CCP Corrective Actions
1. Are specific written corrective actions developed for each CCP in the event that critical limits are not achieved to prevent the release of potentially unsafe food? Will actions be taken to segregate the affected product and assess the safety of the food product to ensure the appropriate disposition?
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - Verification Procedures
1. Will validation of the entire HACCP plan be completed prior to implementation to ensure the elements of the HACCP plan are capable of ensuring control of the significant hazards? This includes validation of the identified hazards, critical control points, critical limits, control measures, frequency and type of monitoring of CCPs, corrective actions, frequency and type of verification and the type of information to be recorded. Guidance note: validation of control measures and their critical limits is completed during the development of the HACCP plan. Validation may include a review of scientific literature, in-house validation studies, and/or using guidancedeveloped by external authorities.
|
Photo
Comment
|
2. Will verification procedures be established to confirm that the HACCP system is working effectively? Do these include procedures to verify that the HACCP plan is being followed, that the HACCP system is able to control hazards on an ongoing basis, and show that the control measures are effective to control the hazards as intended?
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - HACCP System Review
1. Will verification activities be completed on a planned, ongoing basis to ensure that the HACCP system remains capable of safe food production? Verification activities may include but are not limited to: • Review of monitoring records to confirm that CCPs are under control • Review of corrective action records, including specific deviations, product disposition, and root cause to determine the cause(s) of the deviation • Calibration or confirmation of the accuracy of instruments used for monitoring and/or verification activities • Observation to confirm control measures are conducted in accordance with the HACCP plan • Product sampling and analysis (e.g. microbiological hazards such as pathogen testing, chemical hazards such as mycotoxins, or physical/foreign matter hazards such as metal fragments), to verify product safety • Environmental monitoring (e.g. Listeria and/or Salmonella) • Review of the HACCP system, including the hazard analysis and the HACCP plan (e.g. internal and/or third-party audits)
|
Photo
Comment
|
2. Will verification include a comprehensive review of the HACCP system annually or when changes occur, to confirm the efficacy of all elements of the HACCP system? The review of the HACCP system shall confirm the following: • Appropriate significant hazards have been identified • Control measures and critical limits are adequate to control the hazards • Monitoring and verification activities occur as planned and are capable of identifying deviations • Corrective actions are appropriate for deviations that have occurred Guidance note: this review can be carried out by individuals within a food business or by external experts.
|
Photo
Comment
|
5. Will the organization monitor the pre-requisite programs and control measures applied to control hazards? Do procedures include a description of the monitoring methods, responsible personnel, frequency and sampling (as applicable), and monitoring records to be kept? Is the frequency of monitoring appropriate to ensure consistent process control?
|
Photo
Comment
|
6. Will the organization undertake verification activities to confirm that GMP procedures have been effectively implemented and that monitoring is occurring as planned and appropriate corrective actions are taken when requirements are not met? Examples of verification activities may include but are not limited to the following: • Review of GMP procedures, monitoring, corrective actions, and records • Review when any changes occur to the product, process, and other operations associated with the organization • Validation of the cleaning program to meet the required cleaning standards • Records of GMP verification activities shall be retained
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - Microbiological And Chemical Testing
Validation Of The HACCP Plan And Procedures For Verification - Shelf-life Testing
1. Will new and re-developed products with a shelf life of less than two (2) years, have a schedule of shelf-life testing documented and implemented? Will the shelf-life testing schedule include the type of testing to be undertaken and shall be carried out after the expiry date of the product? (i.e. not on the date of expiry)? Considerations for shelf-life testing may include, but are not limited to the following: • Where the product can be frozen, as part of the storage instructions, the end-of-shelf-life testing shall be carried out after the end of the frozen period has been reached • Shelf-life tests may include chemical, microbiological, organoleptic, and physical testing (e.g. weight loss during storage) • Where shelf-life limits are being established for new products, the process for determining the shelf life and any assumptions shall be clearly documented • End-of-shelf-life testing results shall demonstrate that the parameters of the product at the end of shelf life continue to meet the finished product specification. If this is not met, corrective action shall be taken
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - Finished Product Assessments
Validation Of The HACCP Plan And Procedures For Verification - Monitoring And Corrective Actions Of Verification Activities
Validation Of The HACCP Plan And Procedures For Verification - Customer Complaints
Establish HACCP Plan Documentation
1. Is a system of record-keeping relevant to the HACCP system documented and implemented? Are all records associated with the HACCP system retained including: • Monitoring of CCPs • Corrective actions taken regarding CCPs • Changes to the HACCP system • Pre-requisite programmes • Procedures for verification • Validation of critical limits
|
Photo
Comment
|
Personal Hygiene
1. Will a personal hygiene policy and procedure be developed, documented, and implemented? As a minimum, will the following elements be included: Personnel illness and injury (it may be appropriate for personnel to be excluded for a specific time after symptoms resolve or to obtain medical clearance before returning to work): • Eating, drinking, smoking and vaping restrictions • Hand-washing requirements • Hygienic behaviours when sneezing, coughing and blowing of nose • Protection of cuts and wounds and bandage requirements • Clothing and Personal Protective Equipment (PPE) requirements • Jewellery restrictions (including watches and piercings) • Control of personal items including medication and mobile phones • False nails (including acrylics) and false eyelashes • Personnel movement restrictions • Control of visitors and contractors • Procedures to ensure the storage of protective clothing worn in areas of different hygiene risks is not contaminated • Protocols for returning to work after breaks • Use of signs in the language spoken by employees, located in prominent and sensible locations and made of suitable materials to prevent the risk of product contamination
|
Photo
Comment
|
Cleaning
1. Will the organization develop, document, implement and maintain a cleaning program to remove food residues that may be a source of contamination, including allergens? Will cleaning be carried out using wet or dry cleaning methods, (e.g. heat, scrubbing, turbulent flow, vacuum) and chemical methods using solutions of detergents, alkalis, or acids? Will cleaning methods and materials be appropriate to the food type and the surface to be cleaned? The program shall identify the following (where appropriate): • Areas within and outside the building that require cleaning • Equipment that requires cleaning (including cleaning equipment and waste) • Between batch cleaning • Method of cleaning and, where required, sanitation (disinfection) • Frequency of cleaning • Chemicals used, if applicable (all cleaning chemicals shall be approved for use within a food production facility) • Chemical concentrations, contact time and temperature • Persons responsible for cleaning • Records of the monitoring of cleaning • Appropriate training for cleaning personnel
|
Photo
Comment
|
2. Will the cleaning program state how monitoring of cleaning is undertaken, the frequency of monitoring, and corrective action to be taken if monitoring reveals that the cleaning is not effective? Guidance note: Microorganisms may become tolerant to sanitizing (disinfecting) agents over time. A periodic review with the organization’s chemical supplier(s) should be completed to ensure the sanitizer(s) (disinfectant(s)) used are effective to ensure the inactivation of different types of microorganisms (e.g. bacteria and fungi).
|
Photo
Comment
|
4. Is environmental monitoring (e.g. protein and allergen test swabs or microbiological testing for indicator organisms) to validate the effectiveness of the cleaning program undertaken commensurate with product and process risk? Are records of sampling locations, methodology, corrective actions, and retests of sampling locations maintained?
|
Photo
Comment
|
Approved Supplier Programme
2. Will the approved supplier program include criteria for: • Selecting and approving suppliers and service providers • Emergency suppliers/providers • Removing suppliers/providers • Records of approval may include evidence of regulatory compliance, certificates of food safety certification, supplier questionnaires and other formal agreements
|
Photo
Comment
|
3. Will the method of monitoring incoming products and services be documented and implemented and records maintained? Do methods of monitoring include but are not limited to: • Visual inspection to check for packages damaged during transportation, sufficient use-by-date or best-before-date, contamination with foreign matter or allergens during transit, and correct temperature for refrigerated and frozen foods • Receipt of a Certificate of Analysis or other details of compliance to specification • Reconciliation of purchasing documentation for supplier details, date of receipt, and quantity • Incoming materials that do not meet food safety criteria should not be accepted by the organization
|
Photo
Comment
|
Specifications
Labelling
1. Will the organization ensure there is a process for the preparation and review of labels which includes: • Confirmation that the information on the label complies with food safety regulations and other applicable regulations that may apply to specific industry sectors in the country of sale • Confirmation that clear instructions have been provided to enable the next person in the food chain to handle, display, store, and use the product safely • Review of label information in the event of the following: 1. Changes in labeling laws and regulations 2. Changes in raw materials and recipes including the introduction of ingredients that contain allergens applicable in the country of sale 3. Changes in processing that may impact the food safety of the finished product (e.g. change from pasteurization to high-pressure processing) • The label shall be checked prior to production commencing to confirm the correct label, correct date coding for use by/best before date, and legibility.
|
Photo
Comment
|
Allergen Management Program
1. Is an allergen management program documented and implemented to ensure the effective management of allergenic materials to prevent contamination and cross-contact? This program shall include but is not limited to: • A documented risk assessment of ingredients containing allergens (this may form part of the raw material food safety risk assessment) • Receipt and storage practices for ingredients containing allergens • A list of all allergenic ingredients on site • Control measures to prevent contamination and cross contact of allergens in products that do not contain the allergen • Scheduling of production to prevent contamination and cross contact of allergens through shared equipment and processing areas • Policies relating to the use of allergenic ingredients in rework • Consideration of allergens during product development • Mandatory declaration of allergens on product labels as required in the country of sale • Allergen ‘free from’ claims shall be validated and reviewed on an annual basis • Validation and verification procedures for cleaning and maintenance programmes
|
Photo
Comment
|
Packaging
Control Of Non-conforming Product
Traceability
1. Will the organization have a documented procedure that ensures, for all stages of production from receival through to finished goods, products are clearly identified? Will this include (where applicable): • Raw material receival • Storage • Work in progress • Rework • Final product • On hold product • Reject product, quarantined/nonconforming product • Returned product, downgraded/damaged stock • Food waste designated to animal feed • Waste product(s) • Cleaning chemicals and • Packaging • New product development materials
|
Photo
Comment
|
Corrective Action
Recall
2. Will the annual review include a test of the traceability process on at least an annual basis? Will this be performed as a component of the mock recall and will include a test of the forward and backward traceability? (this makes up part of the product traceability exercise referred to in clause 3.9 of this Criteria document).
|
Photo
Comment
|
Design Of Facilities And Equipment - Facility Requirements
Design Of Facilities And Equipment - External Areas
Design Of Facilities And Equipment - Layout, Product Flow And Segregation
2. Will food handling areas that have different levels of hygiene control (e.g. low-risk vs. high-risk areas) shall have appropriate segregation to minimize cross-contamination? Will segregation include walls, partitions, and/or allocation of areas within an open production area and separation in time as appropriate to product and process risks?
|
Photo
Comment
|
Design Of Facilities And Equipment - Building Fabric And Equipment
1. Will the fabrication of the buildings be suitable for the intended purpose and constructed of durable materials that are able to be maintained, effectively cleaned, and where appropriate, sanitized? (disinfected) Will building materials be constructed of non-toxic materials according to the intended use and normal operating conditions?
|
Photo
Comment
|
3. Will floors be impervious to moisture, maintained in good condition, and easy to clean and maintained clean? Where required, will floors be graded to drains to prevent pooling? Where required for wet cleaning operations, will coving between the floor and wall be used to facilitate cleaning?
|
Photo
Comment
|
8. Doors into production areas shall be close-fitting to prevent pest and dust ingress. Doors (including rapid roller doors) shall be kept closed at all times when not in use. Doors that operate as an airlock should not allow for both doors to be open at the same time as this would compromise airlock controls intended to minimize contamination.
|
Photo
Comment
|
Design Of Facilities And Equipment - Employee Amenities
1. Will employee amenities be suitably located and include as required, designated areas for employees to keep personal belongings, changerooms, toilets, hand-washing and drying facilities as well as areas for eating, drinking, and smoking? These facilities shall not be used for other purposes such as storage of food or items that contact food.
|
Photo
Comment
|
Receival And Storage
Dispatch And Transport
Control Of Water, Ice, Air And Other Gases
Control Of Foreign Materials
1. Will controls for foreign materials in food handling areas (e.g. glass, metal, hard and soft plastics, wood splinters, jewelry) shall include suitable prevention strategies including preventative maintenance and regular inspection of equipment? Will procedures for the control of foreign materials be documented, with appropriate records of compliance to procedures retained?
|
Photo
Comment
|
Control Of Chemicals
Maintenance
4. Will maintenance employees and contractors take measures to ensure all tools are suitable for food production areas and that measures are in place to ensure tools and maintenance debris is removed when maintenance activities are completed? This is critical for intrusive maintenance activities where maintenance tools and debris will not be visible to production employees following maintenance activities.
|
Photo
Comment
|
Calibration
Training
1. Will a food safety training program be implemented to ensure personnel handling food have the necessary knowledge and skills? Will the training program consider the food safety knowledge required for the product and process risks including: • Type of food safety hazards known to be associated with the food products handled by the site (e.g. growth of pathogenic or spoilage microorganisms, foreign matter containments, allergens) • The production and packing processes used by the organization
|
Photo
Comment
|
Waste Management
Pest Management
4. Will the program also include: • Bait maps depicting the type and location of treatments • Bait stations shall be secured against movement and tampering • Records of the chemicals used and the concentration • Where required by local regulations, current information for pest control chemicals used or stored on site • If pest control chemicals are stored on-site, these shall be stored in a separate area away from food handling areas and chemicals used for production or maintenance purposes • Where an external pest control contractor is used, evidence of their competency to perform pest inspection and treatment activities shall be maintained • Where pest control activities are carried out by internal personnel, these personnel shall be suitably trained and records of training retained
|
Photo
Comment
|
Management Commitment
1. Does senior management demonstrate commitment to safe food production and handling through: • Promoting food safety awareness throughout the organization? • Facilitating communication relating to food safety issues and incidents? • Providing adequate resources to fully implement the HACCP system to achieve compliance with the BSI HACCP & GMP Certification Criteria?
|
Photo
Comment
|
Continual Improvement
1. Has the effectiveness and continual improvement of the HACCP system been demonstrated through the review of internal verification activities, non-conforming product actions, corrective actions, and the results of external audits? New scientific developments, advances in technology, and industry best practices should also be considered.
|
Photo
Comment
|
Food Safety Policy
1. Will the organization develop a policy that states the organization’s commitment and measurable objectives for the supply of safe and suitable food products that meet customer expectations and legal requirements in the country of manufacture and the country of sale? Will a program to measure and improve food safety culture be established and maintained?
|
Photo
Comment
|
Roles, Responsibilities And Authorities
Controls For Documented Information
1. Has a system to manage documented information (electronic and hardcopy) been implemented to ensure the currency of documentation in use and provide a system for records to be retained and readily retrieved? Documentation and record keeping should be appropriate to the nature and size of the organization and sufficient to verify that the HACCP controls are in place and being maintained. This may include, but is not limited to: • The responsibilities for the development, maintenance, and authorization of all documentation within the HACCP system • Methods of ensuring obsolete documents are removed from use • Responsibilities for the communication of changes to documentation within the HACCP food safety system • Methods for ensuring the security of the documented HACCP food safety system • The method of destruction and control of customer-owned/branded/trademarked documentation, product, and packaging
|
Photo
Comment
|
Document Register
1. Has a document register (list) of the documents referenced in the HACCP system been developed? This may include, but is not limited to the following: • HACCP team composition • Product description and intended use • Hazard analysis, including risk assessment and associated scientific references • CCP determination • Critical limit validation • HACCP audit table • Specifications (raw materials and finished product) • Formulations (recipes) • Prerequisite programmes • Standard operating procedures and work instructions • Policies • Forms
|
Photo
Comment
|
2. Does the organization have access to, and control of, external documents or references required to maintain the HACCP system? This may include but is not limited to food safety statutory and regulatory requirements, codes of practice, guidelines, and standards appropriate to the country in which the food products are manufactured and sold.
|
Photo
Comment
|
HACCP System
The HACCP Team
Scope And Purpose Of The HACCP Plan
2. Will the purpose of the HACCP system include the intent that all food safety hazards will be identified and controlled? Food safety hazards may include but are not limited to: biological, chemical, physical (foreign matter), allergen, and radiological hazards as appropriate to the products in the scope of the HACCP plan.
|
Photo
Comment
|
Product Description And Intended Use
3. A product description for each product or group of products shall detail the following information: Composition (e.g. formulation/ingredients) Physical and chemical characteristics (e.g. final product aW, pH, addition of preservatives) Production methods and technologies (e.g. heat treatment, high-pressure processing (HPP), freezing, drying, brining, etc.) Primary and secondary packaging (e.g. type of packaging used, durability, functional effect on food safety such as the extension of shelf life, etc.) Storage, handling, and distribution methods (e.g. refrigerated/ambient transport requirements) Shelf life (including best-before or use-by-date coding) Intended use of the product(s) Labeling requirements including any claims as per local legislation in the country of sale Allergens as per local legislation in the country of sale Sensitive consumers Some or all of this information may be contained within finished product specifications.
|
Photo
Comment
|
Process Flow Diagram
1. Is a process flow diagram documented for each product or group of products? Is every step in the process(es) identified and sufficiently detailed to include the sequence and interaction of steps, inputs, outsourced processes, intermediate products, rework, end products, waste, and by-products relevant to the process? Complex manufacturing operations may be broken into a series of linked flow diagrams to provide a clear and accurate representation of the process flow.
|
Photo
Comment
|
Hazard Analysis And Control Measures
1. Will a hazard analysis be undertaken and documented for each step of the process and process inputs as identified in the flow process? Will the HACCP team reference the verified process flow diagram in the hazard analysis to identify all potential food safety hazards (as identified in the purpose of the HACCP plan) which need to be prevented, eliminated, or reduced to accepted levels?
|
Photo
Comment
|
3. Are identification and assessment of hazards ungrouped? (e.g. foreign matter which shall be separated into wood splinters, packaging materials, hair, etc.). Is the identification of potential hazards also taken into consideration of the hazards reported in food recalls and outbreaks of foodborne illness as appropriate to the product, process, and global supply chains?
|
Photo
Comment
|
7. When determining significant hazards, does the HACCP team consider the following as applicable to the product and process? • Hazards known to be associated with the type of food, ingredients used in the product, and process steps • Likelihood of occurrence of hazards, taking into consideration prerequisite programs, in the absence of additional control •Likelihood and severity of adverse health effects associated with the hazards in the food in the absence of control • Identified acceptable levels of the hazards in the food (e.g. permissible additives and maximum residue limits defined by regulations in the country of sale) • The food-handling environment and equipment used to produce the food product • The likelihood of survival and/or growth of pathogenic microorganisms • The potential for the presence of toxins (e.g. mycotoxins), chemicals (e.g. pesticides, drug residues), or foreign matter (e.g. glass, metal, soft plastic) • The intended use and/or probability of the product being mishandled by potential consumers that may cause the food to become unsafe Guidance note: There is no specific methodology required to be used to determine the significance of hazards.
|
Photo
Comment
|
8. For all hazards determined to be significant, is there at least one control measure designed to prevent or eliminate the hazard, or reduce the hazard to an acceptable level? Control measures may reference the application of a pre-requisite program to reduce, prevent or eliminate a significant hazard (e.g. cleaning of equipment to prevent cross-contact of food allergens from one food to another food that does not contain that allergen). In other instances, the control measures shall be applied within the process at critical control points (CCPs).
|
Photo
Comment
|
Critical Control Points
1. Will the HACCP team determine the critical control points for hazards identified in the hazard analysis as significant hazards? Will CCPs be established at steps where control is essential to safe food production and where a deviation could result in potentially unsafe food? There may be more than one CCP in a process in which control is applied to address the same hazard. If no control measures exist at any step for an identified significant hazard, then is the product or pro- cess modified? Guidance note: There is no specific methodology required to be used to determine CCPs.
|
Photo
Comment
|
HACCP Audit Table
1. Will a HACCP audit table be developed, documented, and applied which includes all steps of the process where CCPs have been identified? Will the corresponding monitoring activities, corrective actions in the case of deviations, and verification activities be documented for each CCP?
|
Photo
Comment
|
Validated Critical Limits
2. Are critical limits measurable or observable? There may be multiple critical limits identified for a CCP, (e.g. heat treatments may include critical limits for time at temperature). Are critical limits for control measures at each CCP specified and scientifically validated to obtain evidence that they are capable of controlling hazards to an acceptable level if properly implemented? Guidance note: Critical limit criteria may include minimum and/or maximum values (e.g. temperature, time, pH, available chlorine, contact time, conveyor belt speed, flow rate, etc.).
|
Photo
Comment
|
3. Is validation of critical limits considered if the appropriate critical limit has been determined and the capability of the organization to consistently achieve the limit(s)? Validation data shall be documented. Guidance note: validation data may include, but is not limited to: regulations, industry codes of practice, guidance from competent authorities, studies conducted by equipment manufacturers, and site-specific information confirming their capability to consistently achieve the critical limit(s).
|
Photo
Comment
|
System To Monitor Control Of CCPs
3. Will the monitoring procedures for CCPs be capable of timely detection of a deviation from the critical limit to allow non-conforming products to be isolated? The monitoring of CCPs should be continuous, where possible. If monitoring is not continuous, then is the frequency of monitoring sufficient to ensure that the CCP is under control? (e.g. critical limits based on observation such as the application of the correct label to a product containing allergens, needs a monitoring frequency based on the capability of the organization to prevent the distribution of non-conforming products). Guidance note: physical and chemical measurements are usually preferred to microbiological testing because physical and chemical tests can be done rapidly and can often indicate the control of microbial hazards associated with the product and/or the process.
|
Photo
Comment
|
CCP Corrective Actions
1. Are specific written corrective actions developed for each CCP in the event that critical limits are not achieved to prevent the release of potentially unsafe food? Will actions be taken to segregate the affected product and assess the safety of the food product to ensure the appropriate disposition?
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - Verification Procedures
1. Will validation of the entire HACCP plan be completed prior to implementation to ensure the elements of the HACCP plan are capable of ensuring control of the significant hazards? This includes validation of the identified hazards, critical control points, critical limits, control measures, frequency and type of monitoring of CCPs, corrective actions, frequency and type of verification and the type of information to be recorded. Guidance note: validation of control measures and their critical limits is completed during the development of the HACCP plan. Validation may include a review of scientific literature, in-house validation studies, and/or using guidancedeveloped by external authorities.
|
Photo
Comment
|
2. Will verification procedures be established to confirm that the HACCP system is working effectively? Do these include procedures to verify that the HACCP plan is being followed, that the HACCP system is able to control hazards on an ongoing basis, and show that the control measures are effective to control the hazards as intended?
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - HACCP System Review
1. Will verification activities be completed on a planned, ongoing basis to ensure that the HACCP system remains capable of safe food production? Verification activities may include but are not limited to: • Review of monitoring records to confirm that CCPs are under control • Review of corrective action records, including specific deviations, product disposition, and root cause to determine the cause(s) of the deviation • Calibration or confirmation of the accuracy of instruments used for monitoring and/or verification activities • Observation to confirm control measures are conducted in accordance with the HACCP plan • Product sampling and analysis (e.g. microbiological hazards such as pathogen testing, chemical hazards such as mycotoxins, or physical/foreign matter hazards such as metal fragments), to verify product safety • Environmental monitoring (e.g. Listeria and/or Salmonella) • Review of the HACCP system, including the hazard analysis and the HACCP plan (e.g. internal and/or third-party audits)
|
Photo
Comment
|
2. Will verification include a comprehensive review of the HACCP system annually or when changes occur, to confirm the efficacy of all elements of the HACCP system? The review of the HACCP system shall confirm the following: • Appropriate significant hazards have been identified • Control measures and critical limits are adequate to control the hazards • Monitoring and verification activities occur as planned and are capable of identifying deviations • Corrective actions are appropriate for deviations that have occurred Guidance note: this review can be carried out by individuals within a food business or by external experts.
|
Photo
Comment
|
5. Will the organization monitor the pre-requisite programs and control measures applied to control hazards? Do procedures include a description of the monitoring methods, responsible personnel, frequency and sampling (as applicable), and monitoring records to be kept? Is the frequency of monitoring appropriate to ensure consistent process control?
|
Photo
Comment
|
6. Will the organization undertake verification activities to confirm that GMP procedures have been effectively implemented and that monitoring is occurring as planned and appropriate corrective actions are taken when requirements are not met? Examples of verification activities may include but are not limited to the following: • Review of GMP procedures, monitoring, corrective actions, and records • Review when any changes occur to the product, process, and other operations associated with the organization • Validation of the cleaning program to meet the required cleaning standards • Records of GMP verification activities shall be retained
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - Microbiological And Chemical Testing
Validation Of The HACCP Plan And Procedures For Verification - Shelf-life Testing
1. Will new and re-developed products with a shelf life of less than two (2) years, have a schedule of shelf-life testing documented and implemented? Will the shelf-life testing schedule include the type of testing to be undertaken and shall be carried out after the expiry date of the product? (i.e. not on the date of expiry)? Considerations for shelf-life testing may include, but are not limited to the following: • Where the product can be frozen, as part of the storage instructions, the end-of-shelf-life testing shall be carried out after the end of the frozen period has been reached • Shelf-life tests may include chemical, microbiological, organoleptic, and physical testing (e.g. weight loss during storage) • Where shelf-life limits are being established for new products, the process for determining the shelf life and any assumptions shall be clearly documented • End-of-shelf-life testing results shall demonstrate that the parameters of the product at the end of shelf life continue to meet the finished product specification. If this is not met, corrective action shall be taken
|
Photo
Comment
|
Validation Of The HACCP Plan And Procedures For Verification - Finished Product Assessments
Validation Of The HACCP Plan And Procedures For Verification - Monitoring And Corrective Actions Of Verification Activities
Validation Of The HACCP Plan And Procedures For Verification - Customer Complaints
Establish HACCP Plan Documentation
1. Is a system of record-keeping relevant to the HACCP system documented and implemented? Are all records associated with the HACCP system retained including: • Monitoring of CCPs • Corrective actions taken regarding CCPs • Changes to the HACCP system • Pre-requisite programmes • Procedures for verification • Validation of critical limits
|
Photo
Comment
|
Personal Hygiene
1. Will a personal hygiene policy and procedure be developed, documented, and implemented? As a minimum, will the following elements be included: Personnel illness and injury (it may be appropriate for personnel to be excluded for a specific time after symptoms resolve or to obtain medical clearance before returning to work): • Eating, drinking, smoking and vaping restrictions • Hand-washing requirements • Hygienic behaviours when sneezing, coughing and blowing of nose • Protection of cuts and wounds and bandage requirements • Clothing and Personal Protective Equipment (PPE) requirements • Jewellery restrictions (including watches and piercings) • Control of personal items including medication and mobile phones • False nails (including acrylics) and false eyelashes • Personnel movement restrictions • Control of visitors and contractors • Procedures to ensure the storage of protective clothing worn in areas of different hygiene risks is not contaminated • Protocols for returning to work after breaks • Use of signs in the language spoken by employees, located in prominent and sensible locations and made of suitable materials to prevent the risk of product contamination
|
Photo
Comment
|
Cleaning
1. Will the organization develop, document, implement and maintain a cleaning program to remove food residues that may be a source of contamination, including allergens? Will cleaning be carried out using wet or dry cleaning methods, (e.g. heat, scrubbing, turbulent flow, vacuum) and chemical methods using solutions of detergents, alkalis, or acids? Will cleaning methods and materials be appropriate to the food type and the surface to be cleaned? The program shall identify the following (where appropriate): • Areas within and outside the building that require cleaning • Equipment that requires cleaning (including cleaning equipment and waste) • Between batch cleaning • Method of cleaning and, where required, sanitation (disinfection) • Frequency of cleaning • Chemicals used, if applicable (all cleaning chemicals shall be approved for use within a food production facility) • Chemical concentrations, contact time and temperature • Persons responsible for cleaning • Records of the monitoring of cleaning • Appropriate training for cleaning personnel
|
Photo
Comment
|
2. Will the cleaning program state how monitoring of cleaning is undertaken, the frequency of monitoring, and corrective action to be taken if monitoring reveals that the cleaning is not effective? Guidance note: Microorganisms may become tolerant to sanitizing (disinfecting) agents over time. A periodic review with the organization’s chemical supplier(s) should be completed to ensure the sanitizer(s) (disinfectant(s)) used are effective to ensure the inactivation of different types of microorganisms (e.g. bacteria and fungi).
|
Photo
Comment
|
4. Is environmental monitoring (e.g. protein and allergen test swabs or microbiological testing for indicator organisms) to validate the effectiveness of the cleaning program undertaken commensurate with product and process risk? Are records of sampling locations, methodology, corrective actions, and retests of sampling locations maintained?
|
Photo
Comment
|
Approved Supplier Programme
2. Will the approved supplier program include criteria for: • Selecting and approving suppliers and service providers • Emergency suppliers/providers • Removing suppliers/providers • Records of approval may include evidence of regulatory compliance, certificates of food safety certification, supplier questionnaires and other formal agreements
|
Photo
Comment
|
3. Will the method of monitoring incoming products and services be documented and implemented and records maintained? Do methods of monitoring include but are not limited to: • Visual inspection to check for packages damaged during transportation, sufficient use-by-date or best-before-date, contamination with foreign matter or allergens during transit, and correct temperature for refrigerated and frozen foods • Receipt of a Certificate of Analysis or other details of compliance to specification • Reconciliation of purchasing documentation for supplier details, date of receipt, and quantity • Incoming materials that do not meet food safety criteria should not be accepted by the organization
|
Photo
Comment
|
Specifications
Labelling
1. Will the organization ensure there is a process for the preparation and review of labels which includes: • Confirmation that the information on the label complies with food safety regulations and other applicable regulations that may apply to specific industry sectors in the country of sale • Confirmation that clear instructions have been provided to enable the next person in the food chain to handle, display, store, and use the product safely • Review of label information in the event of the following: 1. Changes in labeling laws and regulations 2. Changes in raw materials and recipes including the introduction of ingredients that contain allergens applicable in the country of sale 3. Changes in processing that may impact the food safety of the finished product (e.g. change from pasteurization to high-pressure processing) • The label shall be checked prior to production commencing to confirm the correct label, correct date coding for use by/best before date, and legibility.
|
Photo
Comment
|
Allergen Management Program
1. Is an allergen management program documented and implemented to ensure the effective management of allergenic materials to prevent contamination and cross-contact? This program shall include but is not limited to: • A documented risk assessment of ingredients containing allergens (this may form part of the raw material food safety risk assessment) • Receipt and storage practices for ingredients containing allergens • A list of all allergenic ingredients on site • Control measures to prevent contamination and cross contact of allergens in products that do not contain the allergen • Scheduling of production to prevent contamination and cross contact of allergens through shared equipment and processing areas • Policies relating to the use of allergenic ingredients in rework • Consideration of allergens during product development • Mandatory declaration of allergens on product labels as required in the country of sale • Allergen ‘free from’ claims shall be validated and reviewed on an annual basis • Validation and verification procedures for cleaning and maintenance programmes
|
Photo
Comment
|
Packaging
Control Of Non-conforming Product
Traceability
1. Will the organization have a documented procedure that ensures, for all stages of production from receival through to finished goods, products are clearly identified? Will this include (where applicable): • Raw material receival • Storage • Work in progress • Rework • Final product • On hold product • Reject product, quarantined/nonconforming product • Returned product, downgraded/damaged stock • Food waste designated to animal feed • Waste product(s) • Cleaning chemicals and • Packaging • New product development materials
|
Photo
Comment
|
Corrective Action
Recall
2. Will the annual review include a test of the traceability process on at least an annual basis? Will this be performed as a component of the mock recall and will include a test of the forward and backward traceability? (this makes up part of the product traceability exercise referred to in clause 3.9 of this Criteria document).
|
Photo
Comment
|
Design Of Facilities And Equipment - Facility Requirements
Design Of Facilities And Equipment - External Areas
Design Of Facilities And Equipment - Layout, Product Flow And Segregation
2. Will food handling areas that have different levels of hygiene control (e.g. low-risk vs. high-risk areas) shall have appropriate segregation to minimize cross-contamination? Will segregation include walls, partitions, and/or allocation of areas within an open production area and separation in time as appropriate to product and process risks?
|
Photo
Comment
|
Design Of Facilities And Equipment - Building Fabric And Equipment
1. Will the fabrication of the buildings be suitable for the intended purpose and constructed of durable materials that are able to be maintained, effectively cleaned, and where appropriate, sanitized? (disinfected) Will building materials be constructed of non-toxic materials according to the intended use and normal operating conditions?
|
Photo
Comment
|
3. Will floors be impervious to moisture, maintained in good condition, and easy to clean and maintained clean? Where required, will floors be graded to drains to prevent pooling? Where required for wet cleaning operations, will coving between the floor and wall be used to facilitate cleaning?
|
Photo
Comment
|
8. Doors into production areas shall be close-fitting to prevent pest and dust ingress. Doors (including rapid roller doors) shall be kept closed at all times when not in use. Doors that operate as an airlock should not allow for both doors to be open at the same time as this would compromise airlock controls intended to minimize contamination.
|
Photo
Comment
|
Design Of Facilities And Equipment - Employee Amenities
1. Will employee amenities be suitably located and include as required, designated areas for employees to keep personal belongings, changerooms, toilets, hand-washing and drying facilities as well as areas for eating, drinking, and smoking? These facilities shall not be used for other purposes such as storage of food or items that contact food.
|
Photo
Comment
|
Receival And Storage
Dispatch And Transport
Control Of Water, Ice, Air And Other Gases
Control Of Foreign Materials
1. Will controls for foreign materials in food handling areas (e.g. glass, metal, hard and soft plastics, wood splinters, jewelry) shall include suitable prevention strategies including preventative maintenance and regular inspection of equipment? Will procedures for the control of foreign materials be documented, with appropriate records of compliance to procedures retained?
|
Photo
Comment
|
Control Of Chemicals
Maintenance
4. Will maintenance employees and contractors take measures to ensure all tools are suitable for food production areas and that measures are in place to ensure tools and maintenance debris is removed when maintenance activities are completed? This is critical for intrusive maintenance activities where maintenance tools and debris will not be visible to production employees following maintenance activities.
|
Photo
Comment
|
Calibration
Training
1. Will a food safety training program be implemented to ensure personnel handling food have the necessary knowledge and skills? Will the training program consider the food safety knowledge required for the product and process risks including: • Type of food safety hazards known to be associated with the food products handled by the site (e.g. growth of pathogenic or spoilage microorganisms, foreign matter containments, allergens) • The production and packing processes used by the organization
|
Photo
Comment
|
Waste Management
Pest Management
4. Will the program also include: • Bait maps depicting the type and location of treatments • Bait stations shall be secured against movement and tampering • Records of the chemicals used and the concentration • Where required by local regulations, current information for pest control chemicals used or stored on site • If pest control chemicals are stored on-site, these shall be stored in a separate area away from food handling areas and chemicals used for production or maintenance purposes • Where an external pest control contractor is used, evidence of their competency to perform pest inspection and treatment activities shall be maintained • Where pest control activities are carried out by internal personnel, these personnel shall be suitably trained and records of training retained
|
Photo
Comment
|
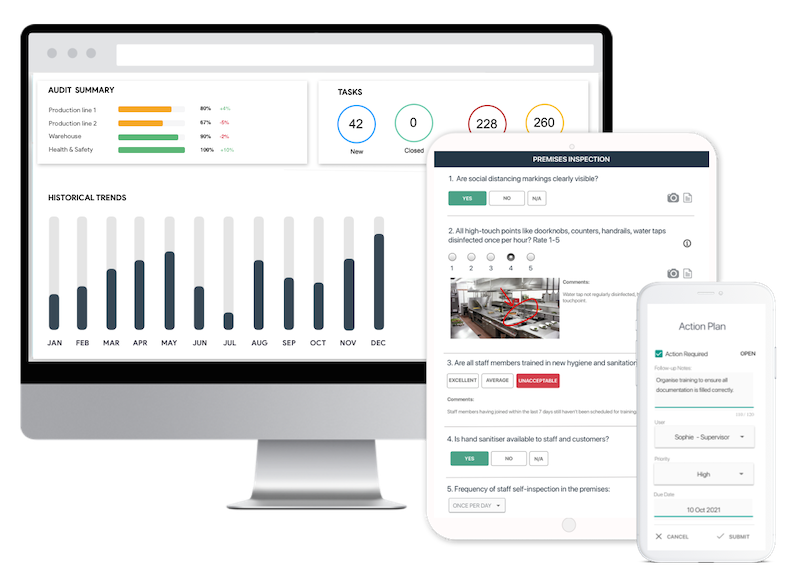
Save Time with Digital Inspections
- Easily capture & attach photos, directly on your mobile device.
- Instantly generate and share detailed reports after the inspection.
- Track corrective actions, view historical trends, improve standards.
What are GMP Checklists?
Good Manufacturing Practices (GMP) audit checklists are systematic tools used to perform GMP audits and ensure compliance with industry regulations and standards in manufacturing processes. These checklists serve as a structured framework to assess whether a company’s operations align with GMP guidelines, which are essential for maintaining product quality and safety. They include a range of areas, including facility conditions, equipment maintenance, personnel training, and documentation practices.
Benefits of Using Digital GMP Checklists
Digital GMP checklists offer numerous advantages over traditional paper-based systems. They facilitate quicker data retrieval, easier sharing of information among team members, and better tracking of compliance trends over time. It not only increases efficiency but also ensures that audit findings are accurately recorded and actionable insights are readily available for decision-making.
Key Elements of GMP Audit Checklists
GMP audit checklists generally cover the following essential elements to ensure comprehensive compliance:
- Facility and equipment cleanliness and maintenance
- Personnel hygiene practices and use of personal protective equipment (PPE)
- Proper handling, storage, and documentation of raw materials and finished products
- Quality control measures and compliance with testing protocols
- Sanitation and cleaning procedures
- Pest control and environmental controls
- Documentation and record-keeping, including batch records and training logs
- Compliance with regulatory and safety standards
- Supply chain management and storage conditions
- Inspection of processing areas, warehousing, and distribution
Different Types of GMP Checklist Templates
Let’s explore different types of GMP audit checklist templates tailored for specific industries and purposes.
GMP Audit Checklist for the Food Industry
In the food industry, GMP standards ensure the safety and quality of food products. A GMP audit checklist typically includes:
- Sanitation practices and personal hygiene protocols.
- Control measures for raw materials and food production processes.
- Monitoring of critical control points (CCPs) to ensure safety.
- Packaging materials and labeling compliance.
- Facility and equipment maintenance.
GMP Pharmaceutical Audit Checklist
The pharmaceutical industry relies heavily on GMP standards to ensure that products are safe, effective, and of high quality. A GMP pharmaceutical audit checklist typically includes sections on:
- Quality Management System (QMS) documentation and updates.
- Training and hygiene practices for personnel.
- Facility and equipment maintenance.
- Batch production records and document control.
- Procedures for raw materials, in-process controls, and product release.
- Quality control in laboratory operations, including testing and standards control.
- Complaint and recall handling.
GMP Warehouse Audit Checklist
Warehouses play a critical role in the supply chain, and a GMP warehouse audit checklist focuses on ensuring that storage and handling practices meet GMP standards:
- Facility layout and sanitation practices.
- Storage conditions for raw materials, intermediates, and finished products.
- Inventory control systems, including tracking and handling of expired materials.
- Security measures to prevent unauthorized access.
- Documentation of storage conditions and inventory transactions.
ISO 22716 Audit Checklist for the Cosmetics Industry
ISO 22716 provides guidelines for the production, control, storage, and shipment of cosmetic products. An ISO 22716 audit checklist includes:
- Compliance with GMP standards specific to cosmetics.
- Documentation of the quality management system.
- Training programs for personnel.
- Facility design, maintenance, and equipment validation.
- Best practices for documentation, including batch records and cleaning logs.
Download for Free GMP Audit Checklist PDF
GMP (Good Manufacturing Practice) Audit
The GMP (Good Manufacturing Practice) audit checklist ensures adherence to high standards in production facilities. It covers various areas such as changing rooms, production areas, CCP checks, chillers, hygiene/washrooms, and staff compliance. Each section includes specific checkpoints, such as verifying water temperature at handwash sinks, ensuring cleanliness and proper use of equipment, and maintaining segregation in chillers.
The GMP Inspection Checklist is designed to help facilities comply with stringent manufacturing standards, focusing on critical areas that impact product quality and safety. It includes detailed checks for proper storage, equipment maintenance, and hygiene practices. Key aspects involve verifying the cleanliness of production areas, ensuring proper labeling and segregation of materials, and maintaining operational equipment.
The GMP Checklist for Breweries is tailored to address the unique needs of the brewing industry. It ensures adherence to good manufacturing practices specific to brewing operations. It covers crucial areas such as ingredient storage, fermentation, packaging, and sanitation. It includes checks for maintaining proper temperatures, ensuring the cleanliness of equipment, and verifying that all brewing processes comply with safety standards.
21 CFR Part 110 – GMP Checklist
This checklist is based on 21 CFR Part 110, which lays out the FDA’s requirements for current Good Manufacturing Practices (cGMP) in food facilities. It helps you systematically audit and ensure compliance across key areas, such as the plant environment and grounds, personnel hygiene, equipment design and sanitation, and production processes. You can use this checklist to confirm that food is safely manufactured, handled, stored, and transported without contamination.
GMP Audit Checklist for Cosmetics
This GMP audit checklist is specifically designed for the cosmetics industry. It can help you inspect and verify compliance across key areas such as buildings and facilities, equipment hygiene, personnel training and cleanliness, raw-material handling, production processes, laboratory testing, record-keeping, labeling, and customer complaints.
GMP Audit Checklist for Pharma
This GMP checklist is tailored for pharmaceutical facilities, covering organisational structure and quality unit responsibilities, document control and GMP SOPs, employee training, plant safety and security, internal audit programmes, and cost of quality. It also addresses facility design, equipment qualification, calibration, material sourcing and handling, process validation, in-process inspections, reprocessing, finished product testing and release, distribution, and complaint handling. It can help comply with regulatory GMP standards throughout the drug manufacturing lifecycle.
Other Popular GMP Templates
Digitize your GMP Audits
- Easily perform audits anywhere using a mobile device, even offline. Capture photos as proof of compliance or areas needing attention.
- Generate instant professional reports with scores and trends, share with stakeholders instantly.
- Assign tasks, set deadlines and track progress, all within a single app
- Analyze data from audits to identify trends, pinpoint recurring issues, and assess compliance levels.