Corrective and preventive action (CAPA) processes are essential to quality management systems (QMS) in regulated industries. CAPA ensures that organizations not only address root causes of nonconformities but also implement systematic actions to prevent recurrence or potential future issues. However, even the best CAPA system needs validation. That’s why a CAPA audit is essential to ensure whether corrective actions are effective, preventive measures are in place, and compliance risks are under control.
This article will cover what CAPA audits are, what triggers them, the steps to perform one, and more. We have also listed free checklists to perform these audits quickly and more efficiently.
What are CAPA Audits?
CAPA audits are formal evaluations of an organization’s corrective and preventive action (CAPA) processes, conducted to ensure that issues are properly identified, investigated, resolved, and prevented from recurring.
They frequently initiate CAPA investigations when deficiencies are found, making them an important feedback mechanism for ongoing risk management and compliance. CAPA audits validate the effectiveness, timeliness, and completeness of CAPA activities, which are central to quality assurance and regulatory compliance in industries like pharmaceuticals, medical devices, and manufacturing.
The four CAPA phases are identification (define the problem), evaluation (assess its impact), investigation (determine the root cause), and implementation (apply corrective and preventive measures). This structured approach ensures problems are resolved effectively and recurrence is prevented.
Common CAPA Inspection Triggers
CAPA inspections often focus on specific triggers that indicate potential risks to product integrity, regulatory compliance, or customer satisfaction.
- Process Deviations and Non-Conformances: Any deviation from approved procedures or failure to meet defined standards can trigger a CAPA inspection. This includes manufacturing process changes, missed critical steps, or deviations from batch records. Even minor non-conformances can indicate systemic issues that require root cause analysis to prevent recurrence.
- Product Quality Issues and Recalls: Defects in product performance, stability failures, or safety concerns can prompt an immediate CAPA review. Regulatory authorities treat recalls as high-risk events, often leading to detailed inspections to verify the adequacy of corrective measures and ensure preventive systems are in place to protect consumers.
- Customer Requests: Complaints, inquiries, or formal requests from customers can initiate CAPA activities. These triggers often highlight quality gaps, unmet specifications, or recurring issues that internal monitoring may have missed.
- Out-of-Specification (OOS) Results: Analytical test results falling outside defined acceptance criteria are a direct red flag. OOS events require thorough investigation to determine whether the cause is an analytical error, equipment malfunction, or a genuine product defect. Inadequate handling of OOS investigations can lead to significant compliance risks during inspections.
- Audit Findings: They often uncover compliance gaps, documentation errors, or process weaknesses. Each significant finding demands a CAPA to address the root cause. Inspectors evaluate whether corrective actions were implemented effectively and whether preventive controls were strengthened to avoid recurrence.
Types of CAPA Audits
There are different types of CAPA audits that serve specific purposes to ensure compliance, efficiency, and continual improvement.
Internal and External Audits
Internal audits assess your CAPA process against internal quality management policies and procedures. They identify gaps before issues escalate to regulatory findings.
External audits are conducted by independent third parties, such as certification bodies or regulators, to verify compliance with industry standards and contractual requirements.
Both types verify whether CAPA records, root cause analyses, and implemented actions align with documented procedures.
Regulatory Audits
These audits are performed by government agencies or regulatory bodies such as the FDA, EMA, or ISO certification auditors. The focus is on verifying compliance with relevant laws, industry regulations, and international standards. CAPA documentation is reviewed for timeliness, traceability, and adequacy of implemented actions. Nonconformities identified here can lead to penalties, recalls, or certification loss.
CAPA Effectiveness and Process Audits
Effectiveness audits confirm whether implemented CAPA actions have resolved the root cause and prevented recurrence. They review post-implementation data, performance metrics, and follow-up monitoring.
Process audits assess the CAPA system’s overall structure, ensuring investigations, risk assessments, and verifications follow standard protocols. They reveal whether the CAPA framework supports continuous improvement.
Supplier Audits
They evaluate a supplier’s CAPA process to ensure product quality and regulatory compliance across the supply chain. These audits review supplier corrective action responses, timelines, and preventive measures.
Regular supplier audits reduce risks of defective materials, delivery delays, or compliance breaches. They also help align supplier practices with your organization’s quality expectations.
Regulatory Requirements: What are the 7 Steps of CAPA
The seven steps of the CAPA process are:
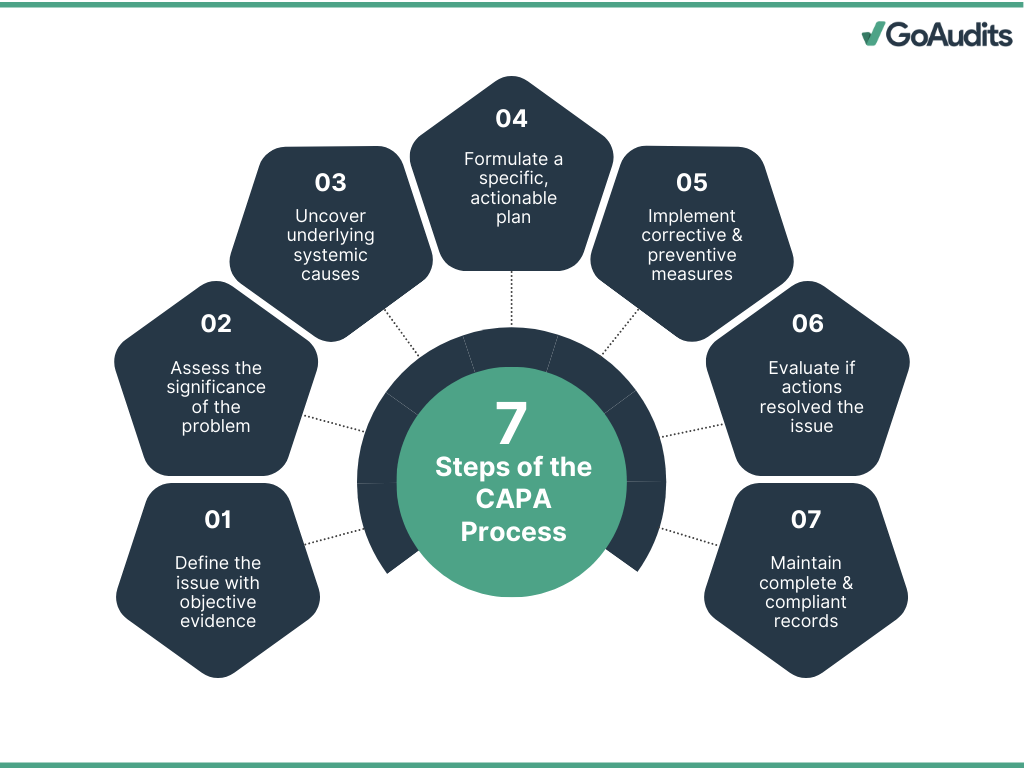
1. Identify the Problem
Define the issue clearly with objective evidence. Regulatory bodies such as the FDA, ISO, EMA, and ICH require organizations to maintain a traceable record of identified nonconformities. Documentation should include audit findings, incident reports, and quality metrics. FDA 21 CFR Part 820.100 and ISO 13485 mandate that records specify the nature of the nonconformance, its source, and its potential impact on safety or operations. Documentation must be precise, dated, and linked to supporting data to withstand regulatory scrutiny.
👉 For more information on FDA guidelines, check out these resources: eCFR: 21 CFR 820.100 – corrective and preventive action and Corrective and Preventive Action Basics.
2. Evaluate and Assess the Problem
Assess the significance of the problem by performing a structured risk assessment. Regulations such as ISO 14971 and ICH Q9 emphasize evaluating severity, occurrence, and detection ratings. This ensures that risks to product quality, compliance, and patient safety are properly prioritized. High-risk issues should receive immediate CAPA attention, as regulators expect evidence-based prioritization.
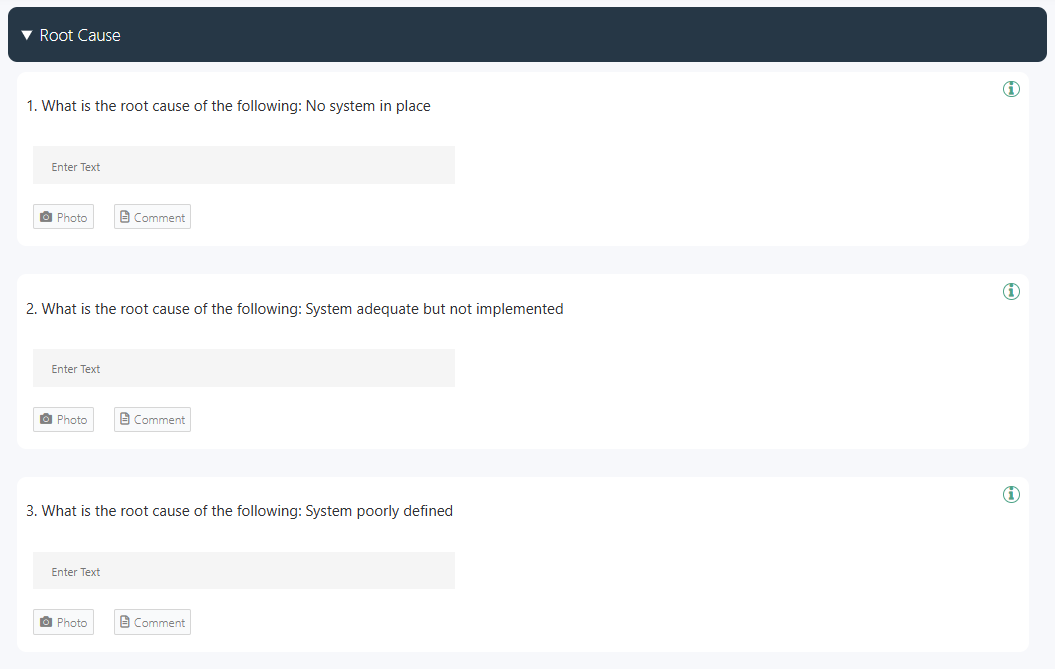
3. Conduct a Root Cause Analysis
Use systematic methods such as the 5 Whys, Fishbone diagrams, or FMEA to uncover underlying systemic causes rather than superficial symptoms. Regulatory frameworks require that RCA findings are documented with evidence, supported by data, and verifiable during audits or inspections. A compliant RCA process must clearly justify conclusions and ensure traceability of decisions.
👉 Use this free GoAudits root cause analysis checklist to identify the reasons behind major problems and implement corrective and preventive measures.
4. Develop an Action Plan
Formulate a specific, actionable, and measurable plan to address the problem and prevent recurrence. Plans must include defined responsibilities, timelines, resources, and acceptance criteria. Regulatory expectations (e.g., FDA guidance) stress alignment between risk assessments and CAPA plans. Preventive measures should be integrated to avoid similar issues across the organization. Verification steps must be included to confirm the effectiveness of actions.
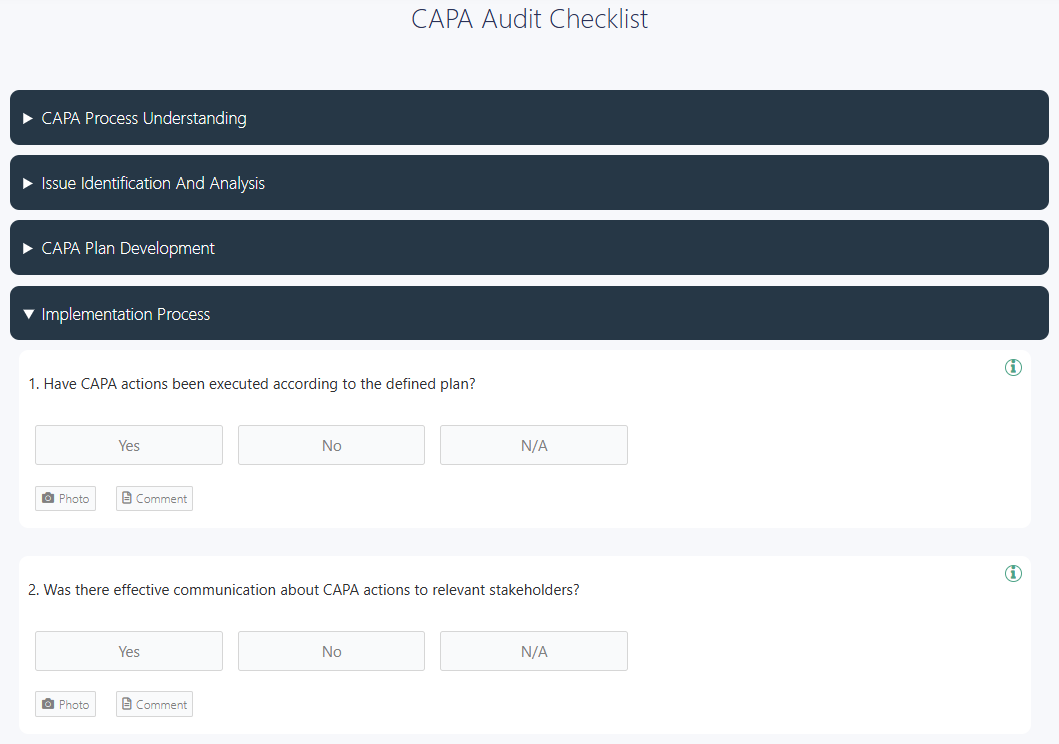
5. Implement the Action Plan
Carry out corrective and preventive measures in line with documented requirements. Ensure proper training, resource allocation, and communication across relevant functions. ISO 9001 and ISO 13485 encourage linking audit management systems with CAPA tracking tools, creating a closed-loop process where audit outcomes automatically trigger CAPA. Real-time data sharing and cross-functional collaboration improve compliance and effectiveness.
6. Verify Effectiveness
Evaluate whether implemented actions have successfully resolved the issue and prevented recurrence. Regulators expect documented verification steps and measurable outcomes. Performance reviews, audits, and monitoring should provide evidence that CAPA actions achieved intended results. Validation must demonstrate both correction of the current issue and mitigation of potential future risks.
7. Document and Close the CAPA
Maintain complete, accurate, and compliant records of the CAPA process. Documentation must be accessible to authorized personnel during inspections, but safeguarded against unauthorized access. Standards such as ISO 27001 require controlled permissions and secure document management to ensure confidentiality and data integrity. CAPA closure should include final approval, ensuring all evidence supports effective problem resolution and compliance with regulatory requirements.
How to Perform an Internal CAPA Audit
An internal CAPA audit ensures that issues are identified, addressed, and prevented effectively. To achieve accurate results, follow a structured and detailed approach.
1. Plan the Audit
Start by defining the audit scope, determining whether you will review the entire CAPA process or focus on specific areas, and set clear objectives. Decide the audit frequency based on regulatory requirements, business needs, and past audit performance. Assemble a qualified audit team with relevant experience, ensuring independence from the areas being audited.
2. Prepare for the Audit
Review relevant documentation, including previous audit reports, CAPA logs, and process maps. Notify the relevant departments in advance, allowing them to prepare records and personnel. Examine CAPA procedures for alignment with internal policies and external regulations. Evaluate open and closed CAPA requests to identify potential focus areas during the audit.
3. Perform the Audit
Conduct interviews with personnel involved in initiating, approving, and implementing CAPA actions. Examine CAPA records for completeness, accuracy, and timely closure. Verify compliance with internal procedures and regulatory requirements. Observe CAPA-related activities to confirm that corrective and preventive measures are applied in practice. Assess effectiveness by checking if root causes were correctly identified, actions were proportionate to the issue, and recurrence was prevented.
👉 Use this free CAPA audit checklist to perform a CAPA audit, evaluate the effectiveness of collective and preventive measures, and ensure the quick resolution of issues.
4. Report the Audit Findings
Document all findings clearly and objectively. Include nonconformities, observations, and best practices. Provide evidence for each finding and link them to the applicable procedure or regulation. Prioritize findings by risk level to ensure timely action on critical issues.
Learn More: How CAPA Software Can Simplify the Process of Reporting CAPA Audits
5. Monitor and Follow Up
Assign responsibilities for corrective actions to relevant personnel. Define realistic deadlines and track progress until completion. Verify the implementation and effectiveness of corrective measures through follow-up checks. Close the audit only when all actions are completed and verified.
Leverage GoAudits to Perform CAPA Inspections Up to 5x Faster
GoAudits is a comprehensive audit and inspection management software. It’s designed to streamline compliance, quality checks, and operational oversight. GoAudits can help you streamline the entire CAPA process so you can resolve issues promptly, maintain compliance, and prevent recurrence.
- Mobile inspections: Perform CAPA audits anytime, anywhere, even offline.
- Instant CAPA assignment: Assign corrective actions during the audit with deadlines and accountability.
- Automated audit reports: Generate audit reports, rich with photo evidence, timestamps, geolocation, corrective actions, etc., instantly.
- Digital CAPA audit reports: Ensure compliance and traceability with real-time updates.
- Data analytics & dashboards: Identify recurring issues and track performance trends.
- Free templates: Access industry-specific CAPA audit checklists.
Common Issues Found in CAPA Audits
CAPA audits often reveal recurring CAPA weaknesses:
- Poor Root Cause Analysis: You may see teams identifying only superficial symptoms instead of underlying causes. Without a thorough investigation, corrective actions target the wrong issues, leading to repeated problems.
- Ineffective CAPA Action Plans: Action plans often lack clarity, timelines, or measurable outcomes. In some cases, responsibilities are not assigned, or milestones are missing, making it difficult to track progress or ensure timely closure.
- Poor or Complex CAPA Management Processes: Overly complicated CAPA workflows slow down implementation. Conversely, overly simplified processes miss critical steps. Inefficient systems lead to bottlenecks, overlooked actions, and poor oversight.
- Inadequate Training: Employees involved in CAPA may lack the skills to conduct investigations, perform risk assessments, or use CAPA tools effectively. Without targeted training, execution quality suffers and compliance risks increase.
- Poor Documentation: Incomplete, inconsistent, or disorganized CAPA records are a common audit finding. Missing evidence of investigations, approvals, or verification steps raises questions about process integrity and regulatory compliance.
- Lack of Communication and Collaboration: CAPA activities often span multiple departments. Without clear communication channels, teams operate in silos, leading to duplicated work, missed deadlines, or conflicting actions.
- Reactive Instead of Proactive Approach: A CAPA system that responds only to problems, rather than anticipating them, limits its value. Auditors frequently note the absence of trend analysis, risk monitoring, or early detection mechanisms.
- Insufficient Verification of Effectiveness: Some CAPAs are closed without verifying whether implemented actions actually prevent recurrence. Lack of follow-up or premature closure leads to recurring nonconformities and wasted effort.
- Lack of Risk Assessment: Risk assessment is often overlooked during CAPA planning. Without prioritizing actions based on potential impact, critical issues may receive insufficient attention while low-risk items consume resources.
Key Objectives & Benefits of CAPA Audits
A CAPA audit offers measurable benefits for your organization:
- CAPA audits confirm that your processes meet relevant industry regulations and standards. They identify gaps in compliance early, reducing the risk of legal penalties, product recalls, or certification loss.
- An effective CAPA audit assesses how well corrective and preventive actions integrate into your QMS. It ensures processes are consistent and aligned with international quality standards such as ISO 9001 or ISO 13485.
- By tracking nonconformities and addressing root causes, CAPA audits directly enhance product reliability and service consistency. This leads to fewer defects, rework, and warranty claims.
- Audits verify that customer complaints and feedback are properly addressed. This proactive approach builds trust, increases repeat business, and strengthens your brand reputation.
- CAPA audits ensure that corrective and preventive measures deliver the intended results. They validate that problems are permanently resolved rather than temporarily patched.
- Identifying process inefficiencies during a CAPA audit reduces waste, streamlines workflows, and optimizes resource allocation. This leads to faster turnaround times and improved productivity.
- Regular CAPA audits provide valuable data for trend analysis, enabling your team to anticipate risks and implement preventive strategies before issues escalate.
- CAPA audits ensure that every CAPA process is well-documented, creating a clear trail for internal reviews and external inspections. Accurate records support transparency and traceability across operations.
- By addressing issues early and preventing recurrence, CAPA inspections minimize costs associated with defects, rework, downtime, and noncompliance penalties.
CAPA audits are essential to maintaining compliance, improving quality, and preventing recurring issues. By planning audits carefully, using structured checklists, and leveraging digital tools like GoAudits, organizations can significantly speed up CAPA inspections while maintaining accuracy and accountability.