HIPAA’s regulatory framework was created to protect the confidentiality and integrity of patient data, and the Office for Civil Rights is the primary enforcer of those standards. When OCR encounters complaints, breach reports, or evidence of systemic gaps, it can trigger an investigative process that ranges from formal audits to substantial civil penalties. Repeated violations or willful neglect can lead to fines, mandatory corrective action plans, and even criminal referrals.
Covered entities and business associates must treat compliance as an ongoing process. Regular internal HIPAA audits, continuous review of audit trails, and timely detection of unauthorized activity are essential. This article will explore different types of HIPAA audits, how to prepare for them, and how HIPAA audit software and automated audit simplify monitoring, maintain required documentation, and demonstrate compliance readiness if OCR initiates an investigation.
What are HIPAA Audits?
HIPAA audits are formal reviews conducted to assess an organization’s compliance with the Health Insurance Portability and Accountability Act (HIPAA) regulations, particularly focusing on the Privacy, Security, and Breach Notification Rules. Audits can be conducted internally or by third parties, but federal enforcement audits are carried out by the Department of Health and Human Services (HHS) Office for Civil Rights (OCR).
OCR uses a standardized HIPAA audit program and audit protocol to evaluate policies, technical safeguards, risk analysis, and real-world operations. At the same time, covered entities and business associates are expected to perform their own internal audits and evaluations of their safeguards.
Who is Required to Perform HIPAA Audits
HIPAA requires covered entities, such as healthcare providers, health plans, and healthcare clearinghouses, as well as their business associates (third-party vendors handling protected health information), to perform HIPAA audits. This includes conducting both external audits (when selected by OCR) and regular internal audits to maintain compliance.
When and How Often are These Audits Performed
HIPAA audits do not follow a rigid, fixed schedule. OCR may conduct audits periodically, triggered by different reasons, with no set annual cycle. However, HIPAA regulations require covered entities and business associates to conduct their own internal audits at least annually to assess administrative, technical, and physical safeguards. Many organizations choose to audit more frequently (quarterly or biannually) depending on regulatory changes, technological advancements, and risk assessments.
Why are HIPAA Compliance Audits Performed?
Here are some reasons why it’s important:
- Validate whether an organization’s privacy and security practices meet HIPAA’s mandatory requirements and reveal gaps that must be corrected.
- Confirm that technical, administrative, and physical safeguards are functioning as intended, reducing vulnerabilities that could compromise the confidentiality, integrity, or availability of protected health information.
- Identify exposure points that could lead to data breaches, civil penalties, or corrective action plans, enabling proactive remediation before issues escalate into costly enforcement actions.
- Demonstrate a visible commitment to protecting patient information, reinforcing confidence among patients, partners, and regulators while strengthening the organization’s overall reputation in the healthcare market.
Types of HIPAA Compliance Audits
HIPAA compliance audits fall into several distinct categories, each serving different purposes and operating under different protocols.
HIPAA Self-Audits
HIPAA self-audits are internal assessments that covered entities and business associates perform to test their own compliance with HIPAA rules. There is no requirement to hire an independent auditor, and HHS does not recognize any official ‘HIPAA certification’.
OCR guidance and industry best practice treat annual self-audits as the minimum baseline for demonstrating due diligence.
These mandatory HIPAA self-audits include:
- Security risk analysis (IT risk assessment)
- Privacy standards audit
- Security standards audit
- HITECH Subtitle D audit
HIPAA IT Audits
HIPAA IT audits evaluate an organization’s cybersecurity and data protection measures, specifically examining how technical controls safeguard patient data. These audits assess technical, physical, and administrative controls to ensure ePHI and PHI maintain availability, integrity, and confidentiality.
The IT audit examines four primary categories required under the Security Rule:
- Access controls
- Audit controls
- Integrity controls
- Transmission security
HIPAA Security Audits
A HIPAA security audit is a holistic evaluation of an organization’s overall security posture and its ability to protect patient information across all domains. The US Health and Human Services Office or independent auditors conduct these assessments to verify compliance with HIPAA Security Rule requirements.
Assessment areas include:
- Administrative safeguards
- Physical safeguards
- Technical safeguards
HIPAA Privacy Audits
HIPAA Privacy Rule audits focus specifically on the two basic requirements: protecting individually identifiable health information from impermissible uses and disclosures, and ensuring individuals retain rights over their protected health information.
Core audit requirements:
- Confirm documented Privacy Rule–compliant policies for PHI use, disclosure, minimum necessary, and data accuracy.
- Validate procedures and timely responses for access, amendments, restrictions, and confidential communications.
- Ensure complete logs of PHI disclosures and the ability to provide accountings when required.
- Review breach detection, investigation, notification processes, and supporting documentation.
- Verify periodic and new-hire Privacy and Breach Rule training with recorded completion.
- Confirm executed Business Associate Management (BAAs) defining PHI use, safeguards, incident reporting, and subcontractor obligations.
HIPAA OCR Audits
The Office for Civil Rights has the authority to audit any healthcare-covered entity or business associate to ensure compliance with the HIPAA requirements.
👉 Every covered entity and business associate is eligible for a HIPAA audit, regardless of whether complaints have been filed.
OCR audits are triggered through multiple pathways:
- Patient or Employee Complaints: OCR investigations most often begin with complaints alleging HIPAA violations, including privacy breaches, unauthorized PHI access, or retaliation against whistleblowers.
- Reported Data Breaches: Breaches affecting 500+ individuals trigger automatic OCR review; smaller breaches may also be investigated based on severity or compliance history.
- Random OCR Audits: OCR conducts proactive audits by randomly selecting entities based on size, type, geography, and enforcement priorities.
- Whistleblower Reports: Employees or contractors may report improper PHI practices, prompting OCR investigations.
Here are the key differences between internal HIPAA audits and OCR audits:
| Dimension | Internal HIPAA Audits | OCR Audits |
| Authority and Enforcement | Conducted voluntarily by the organization with no external enforcement power. Outcomes guide internal improvement and readiness efforts. | Conducted under federal authority by HHS OCR, which can investigate violations, mandate corrective actions, and levy civil or criminal penalties for serious noncompliance. |
| Scope and Standardization | Flexible in scope, depth, and methodology. Teams can tailor reviews to operational priorities or resource constraints. | Uses OCR’s standardized Compliance Audit Protocol with defined modules for the Privacy, Security, and Breach Notification Rules. Protocol includes testable requirements, evidence expectations, and uniform evaluation criteria. |
| Objectivity and Rigor | May reflect internal biases, institutional familiarity, or incomplete visibility into control gaps. | Performed by external auditors applying consistent methods that strengthen objectivity and surface issues organizations may overlook. |
| Documentation Expectations | Basic documentation is often sufficient: policies, procedures, and select operational artifacts. | Requires evidence-based documentation demonstrating that controls are implemented and operating. OCR typically requires at least two artifacts showing control performance over time. |
| Response and Remediation | Results in internal recommendations; corrective actions are self-directed and self-monitored. | Generates formal draft and final reports. Entities must provide written responses, remediation commitments, and demonstrate implementation under OCR oversight. |
| Gap Assessment vs. Risk Assessment Relationship | Identify compliance shortfalls, but can be limited to checklist-style comparisons. | Never replace a full risk analysis, which must assess threats, vulnerabilities, and impacts unique to the organization’s environment. |
How to Prepare for HIPAA Compliance Audits
The following points outline a framework that organizations can use to strengthen readiness and reduce audit exposure.
1. Understand HIPAA Audit Requirements
Auditors evaluate how well an organization implements HIPAA requirements, focusing on:
- Governance: Leadership oversight, assigned security responsibilities, and accountability structures.
- Risk management: Existence and quality of risk analysis and risk mitigation plans.
- Access controls: Authentication, authorization, user provisioning, and access review processes.
- Technical safeguards: Encryption, activity logs, transmission controls, and system hardening.
- Policies and procedures: Availability, alignment with rule requirements, and evidence of routine review.
- Workforce training: Frequency, scope, and documentation of security and privacy instruction.
- Incident response: Breach identification, reporting timelines, and documentation.
- Business associate oversight: Contracts, due diligence, and monitoring activities.
The audit process generally includes a document request, submission of evidence, remote or onsite review, follow-up questions, and a final audit report.
2. Develop a HIPAA Audit Program
A formal audit program defines audit cycles, control owners, and issue-tracking processes. It aligns compliance, IT, operations, and leadership around clear expectations and ensures audit readiness before external reviews.
3. Conduct a Risk Analysis
Risk analysis should begin with an inventory of systems and workflows that store or interact with PHI. Each asset must be evaluated for vulnerabilities that could compromise confidentiality or integrity. Teams should focus remediation efforts where the organizational risk is highest. Auditors consistently cite outdated or incomplete risk analyses as a major compliance gap, so maintaining a current assessment is critical.
4. Update Policies and Procedures
Policies must accurately reflect day-to-day operations. Review and update documents covering access controls, encryption, remote work, incident response, and workforce privacy. All policies should be current, approved, versioned, and aligned with HIPAA requirements.
5. Review Business Associate Agreements
All entities handling PHI must have current, compliant BAAs. Agreements should address safeguards, breach notification timelines, subcontractor obligations, and data disposition. A centralized BAA inventory demonstrates active third-party oversight.
6. Strengthen Incident Response and Disaster Recovery
Organizations must be able to identify, contain, and report security incidents promptly. Document response procedures, clearly assign roles, test communication channels, and properly implement recovery processes. Disaster recovery should support the timely restoration of systems that handle PHI, with evidence of recent testing available for audit review.
7. Train Employees
HIPAA training should be conducted at least annually and tailored by role. Scenario-based instruction improves awareness of phishing, disclosures, and incident reporting. Completion records must be complete and readily available for audit review.
8. Centralize Evidence
A centralized document repository removes friction and guesswork during audits and shortens auditor review cycles. This repository should include policies, risk analyses, training logs, access records, system logs, encryption validation, backup testing results, and vendor management documentation.
9. Meet HIPAA Audit Trail Requirements
The HIPAA Security Rule requires covered entities and business associates to implement mechanisms that record and examine activity in information systems containing electronic PHI (ePHI). In practice, that means:
- Automatic audit logging of access to ePHI (not just manual logs).
- Core data elements: User ID, date and time, system or record accessed, action taken (view, create, modify, delete, export), and whether the attempt was successful.
- Coverage for key systems: EHR/EMR platforms, messaging tools, file storage, email, and any applications that process PHI.
- Retention: Audit logs and trails must be retained for at least six years, and longer if stricter state laws or internal policies apply.
👉 During audits, OCR often requests examples of audit logs showing how you monitor access, investigate suspicious activity, and document responses.
HIPAA audit software centralizes logs, making it much easier to prove that audit controls are in place and operating.
10. Leverage HIPAA Audit Software & Checklists
HIPAA audit software strengthens consistency and reduces manual effort. Effective platforms allow teams to define audit scope, map controls to HIPAA requirements, validate safeguards, flag operational gaps, and track remediation to completion. HIPAA audit software provides the audit trails, timestamps, and structured evidence that auditors expect. Using these tools also helps organizations measure compliance maturity over time.
11. Document Findings and Corrective Actions in a HIPAA Audit Report
A clear audit report should summarize findings, supporting evidence, root causes, and remediation timelines. Reports should highlight both strengths and gaps, with a focus on actionable improvements.
12. Act on Findings and Maintain Continuous Improvement
Audit findings should drive prioritized remediation and verified closure. Ongoing internal reviews, policy updates, and leadership reporting support continuous compliance and reduce future audit risk.
Perform HIPAA Self-Audits with HIPAA Audit Software
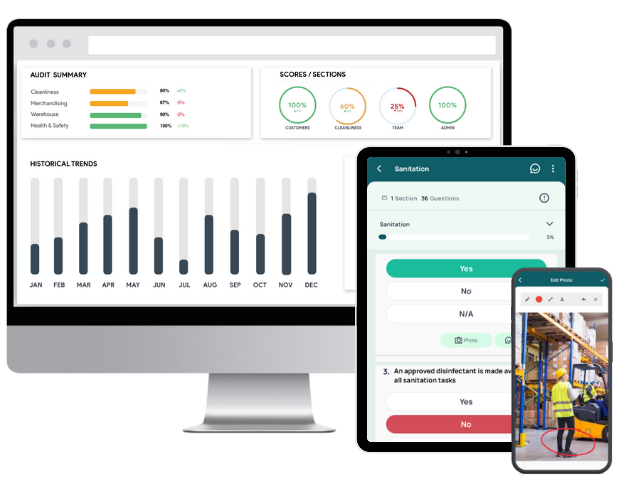
GoAudits gives healthcare organizations a single, reliable system to plan, execute, and monitor internal HIPAA audits. It removes manual complexity by consolidating checklists, documentation, reporting, and corrective actions into one mobile solution. Auditors capture evidence, while automated reporting and smart dashboards surface issues faster. GoAudits healthcare compliance software makes the HIPAA audit process faster, more accurate, and fully traceable.
- Access ready-to-use audit forms, build tailored ones using drag-and-drop tools, or have the GoAudits team digitize your existing forms at no extra cost.
- Perform internal HIPAA audits 5x faster, even offline. Capture photos, e-signatures, timestamps, and geo-location for transparency.
- Generate professional HIPAA audit reports instantly at the end of each audit. Customize report layout and branding to match organizational standards and regulatory documentation needs.
- Access automated scoring, historical comparisons, recurring issues, and progress tracking to identify gaps and monitor improvements over time.
- Assign corrective actions during the audit, set priorities and due dates, and track completion from a centralized dashboard.
- Trigger email alerts for approvers and task owners, and ensure proper escalation, permissions, and visibility for multi-site operations.
- View audit results across locations, teams, and categories. Plan recurring audits, view upcoming ones, detect delays, and supervise follow-up actions in one unified interface.
Free HIPAA Audit Checklists Included in GoAudits
HIPAA compliance spans multiple rules, each with its own expectations, evidence requirements, and safeguards. These healthcare checklists break those requirements into focused, manageable sections so healthcare organizations can evaluate compliance one rule at a time. This makes audits clearer, more consistent, and easier to maintain across the organization:
- HIPAA Compliance Checklist
- HIPAA Omnibus Checklist
- HIPAA Privacy Rule Checklist
- HIPAA PHI Security Breach Report
- HIPAA Security Rule Compliance Checklist
- HIPAA Annual Risk Assessment Checklist
- HIPAA Breach Notification Letter Template
HIPAA Audit Certification: An Overview
HIPAA audit certification involves third-party assessments that evaluate an organization’s readiness for HIPAA compliance.
👉 No organization or third party can issue an official HIPAA certification that is recognized by the US Department of HHS or the OCR. HHS does not provide, endorse, or recognize any specific HIPAA certification for organizations or individuals. Instead, HIPAA compliance is assessed through audits and investigations conducted directly by the HHS OCR.
Third-party audits provide evidence of due diligence but do not guarantee immunity from HHS enforcement.
Cost and Timeline for HIPAA Audit Certification
Costs for a third-party HIPAA audit typically range from $20,000 to $100,000+, depending on organization size, scope, and auditor expertise.
Timelines vary from 2-3 months for smaller entities to 6-12 months for complex operations, including preparation, on-site reviews, and remediation.
Steps to Obtain HIPAA Compliance Audit Certification
- Appoint a dedicated HIPAA security and privacy officer to oversee policies and training.
- Engage an accredited third-party auditor, such as those aligned with HITRUST or ISO standards, for a gap assessment.
- Conduct internal risk analyses, remediate findings via corrective action plans, and undergo the formal audit for an attestation of compliance readiness.
FAQs
The most common HIPAA violation involves impermissible disclosures or uses of PHI, such as unauthorized access to patient records by staff, discussing PHI in public areas, or failing to secure devices containing PHI, which often leads to breaches reported to the HHS OCR. These incidents frequently stem from inadequate employee training or weak access controls, accounting for a significant portion of enforcement actions. Examples include hospitals fined for filming patients without consent or sharing records via unsecured methods.
HIPAA audits strengthen audit programs by systematically evaluating compliance with Privacy, Security, and Breach Notification Rules, identifying gaps in safeguards, policies, and procedures before OCR enforcement occurs. They promote proactive risk management, such as reviewing administrative, physical, and technical controls, which enhances data security and reduces violation risks through documented evidence of due diligence. Regular internal audits also ensure accountability, aligning with HHS Phase 2 protocols that scrutinize real-world implementation.
OCR expects current, approved, and implemented policies plus evidence that policies operate in practice. Requested documentation typically includes:
– Organizational charts and HIPAA Privacy and Security Officer roles
– Documented policies and procedures for Privacy Rule, Security Rule, and Breach Notification Rule compliance
– Privacy Practices Notices and form templates
– Documented policy reviews, approvals, version histories, and workforce acknowledgments
– Minimum necessary procedures and disclosure logs
– Business Associate Agreements and vendor due diligence records
– Security risk analysis and risk management plans
– Workforce training materials, rosters, and completion records
– System logs and audit trails showing control operation over time
HIPAA audit trail requirements mandate covered entities to implement hardware, software, and procedural mechanisms to record and examine ePHI activity in information systems, covering user actions, system events, and application logs. Key elements include tracking access attempts (authorized or not), date/time stamps, user IDs, and PHI details to ensure the minimum necessary standard and enable breach detection. Entities must assess audit capabilities during risk analysis and retain logs per retention policies.
No official entity issues a universally recognized ‘HIPAA certification’ endorsed by HHS, as HIPAA compliance is self-attested through risk analyses and does not involve formal certification by the department. Third-party auditors or HITRUST assessors may provide compliance validations, but HHS recognizes only its own audits and does not accredit external certifications for HIPAA. Organizations should pursue these for credibility while maintaining internal documentation for OCR reviews.
HIPAA audit software simplifies audits by automating log collection, real-time monitoring of access trails, and generating compliance reports, reducing manual review time and errors. It streamlines certification-like processes by mapping controls to HIPAA standards, flagging risks, and supporting risk analyses with dashboards for quicker remediation. They also ensure audit trail integrity across systems, helping business associates in scalable compliance without extensive IT overhauls.