A corrective and preventive action (CAPA) report is your documented proof that problems have been solved at the root, and that measures are in place to prevent them from returning. Whether you operate in manufacturing, healthcare, or food production, regulators such as the FDA require documented CAPA processes, and standards bodies like ISO 9001 set clear requirements for how they should be managed. A well-prepared CAPA report not only demonstrates compliance but also reduces repeat issues, improves quality, and lowers costs.
This article will explore the key elements and steps to prepare a CAPA report, along with free checklists and a CAPA report sample to get you started.
- Understanding Corrective Actions and Preventive Actions
- What is a Corrective Action and Preventive Action Report?
- CAPA Report Template: Key Elements
- Free CAPA Report Sample
- How to Prepare a Corrective and Preventive Action Report?
- Leverage GoAudits for Corrective and Preventive Action Reporting
- Why is a Corrective and Preventive Action Report Important?
- FAQs
Understanding Corrective Actions and Preventive Actions
Corrective actions and preventive actions (CAPA) are central concepts in quality management and risk control systems across industries.
Corrective Actions
Corrective actions are steps taken to eliminate the causes of an existing problem, nonconformity, or undesirable situation to prevent its recurrence. They are reactive, meaning they’re implemented after a problem or error has already occurred. The process starts by identifying the problem, analyzing its root cause, and then creating and implementing solutions that address the source of the nonconformity, ensuring the same issue does not reappear.
Preventive Actions
Preventive actions are proactive measures aimed at identifying and eliminating potential causes of problems, nonconformities, or undesirable situations before they occur. These actions involve risk analysis, data review, and trend assessment to predict possible future issues, followed by changes or controls to prevent the problem from occurring.
| Aspect | Corrective Action | Preventive Action |
| Timing | After a problem has occurred | Before a problem occurs |
| Nature | Reactive | Proactive |
| Focus | Fixing the root cause of the detected nonconformity | Identifying and eliminating potential causes of nonconformity |
| Objective | Prevent recurrence of the same issue | Avoid the occurrence of possible issues |
| Scope | Addresses existing problems | Addresses potential or anticipated problems |
| Process Steps | Root cause analysis, implement solution, monitor | Risk assessment, plan mitigation, and monitor effectiveness |
| Examples | Changing a process after a defect is found | Implementing controls based on risk analysis |
| Methodologies | Problem-solving tools (e.g., 5 Whys, Fishbone Diagram) | Risk assessment (e.g., FMEA, data analysis) |
What is a Corrective Action and Preventive Action Report?
A corrective action and preventive action (CAPA) report is a structured document used to identify, address, and prevent issues that can impact product quality, compliance, safety, or operational efficiency. It records the problem, its root cause, the corrective actions taken to eliminate the issue, and the preventive actions implemented to avoid recurrence.
A CAPA report ensures traceability, accountability, and compliance with regulatory requirements, especially in industries like manufacturing, pharmaceuticals, medical devices, and food processing.
Common situations when a CAPA report is required include:
- Regulatory non-compliance
- Customer complaints
- Process deviations
- Safety incidents
- Recurring problems
- Risk identification
In regulated industries, CAPA reports are often mandatory for demonstrating compliance with ISO standards, FDA requirements, or other quality management frameworks. They act as formal evidence that your organization has addressed the issue, eliminated its cause, and put controls in place to prevent recurrence.
CAPA Report Template: Key Elements
A well-structured corrective action report should have the following key elements.
Identified Issues and Non-Conformances
Start by clearly describing the problem or non-conformance. Specify what happened, when it occurred, and where it was detected. Use objective evidence, such as inspection reports, audit findings, or customer complaints. Avoid assumptions or general statements and establish a factual record of the issue.
Root Cause of the Identified Issues
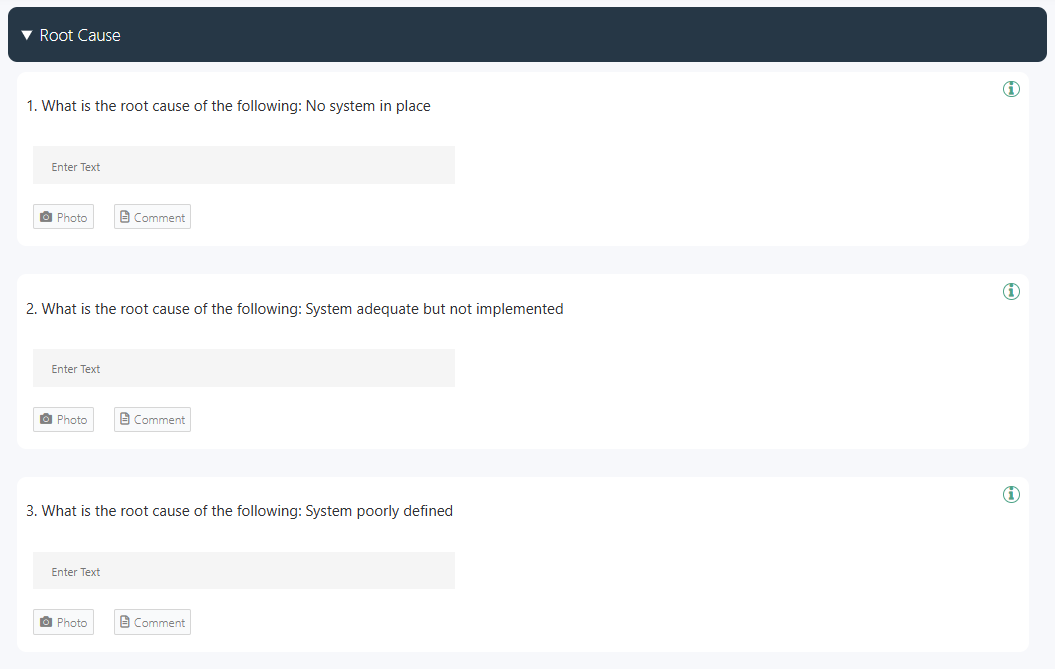
Identify the underlying cause, not just the symptoms. Use root cause analysis techniques such as the 5 Whys or fishbone diagrams. Determine whether the cause is related to processes, materials, equipment, training, or human factors. This ensures that actions address the real problem rather than providing temporary fixes.
👉 You can also use this free root cause analysis checklist offered by GoAudits to identify and investigate the root cause and implement measures to correct and prevent them.
Corrective Action Plan
Outline the specific steps to eliminate the root cause. Assign responsibilities and set realistic timelines for each action. Include details on the resources required, procedural changes, or repairs needed. The plan should be measurable, actionable, and directly linked to the identified cause.
Preventive Action Plan
Preventive actions protect operational consistency and reduce the risk of recurrence. Describe measures to avoid similar issues in the future, such as improved training programs, process redesign, or enhanced monitoring systems.
Implementation and Verification of Effectiveness
Detail how the corrective and preventive actions will be executed. Once implemented, verify their effectiveness through follow-up audits, inspections, or performance metrics. Clearly define the verification method and timeframe to confirm that the issue is resolved and the process is stable.
Documentation
Maintain complete records of the issue, analysis, action plans, and verification results. Documentation supports compliance with quality management standards and provides evidence during audits. Ensure that records are organized, accessible, and regularly updated.
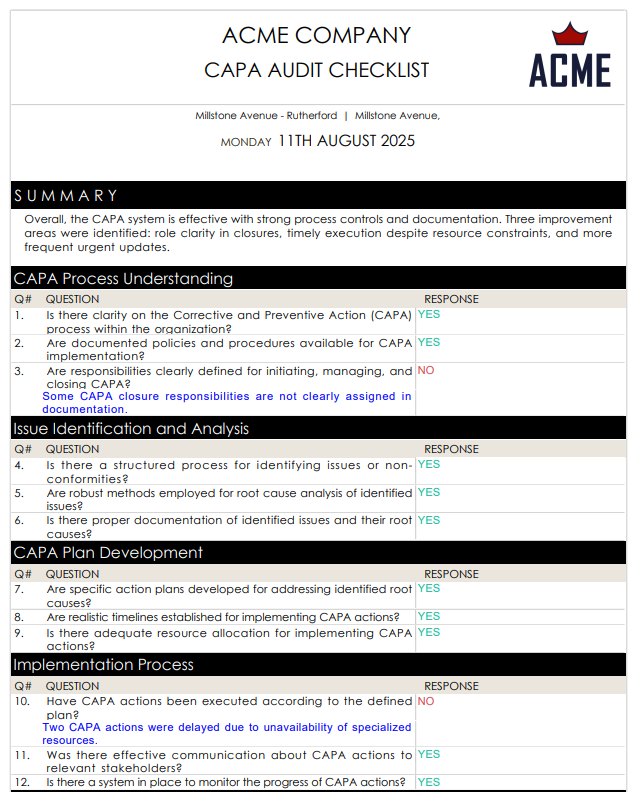
Free CAPA Report Sample
Here’s a free corrective action report sample generated using GoAudits.
How to Prepare a Corrective and Preventive Action Report?
Preparing an effective CAPA report requires a structured, evidence-based approach. Each step must document facts, decisions, and outcomes to ensure compliance, accountability, and continuous improvement.
1. Use Digital Checklists to Perform an Audit
Begin with a thorough audit using standardized digital checklists. These ensure all inspection points are covered consistently and that data is captured in real time. Include photographs, timestamps, and relevant references for complete documentation.
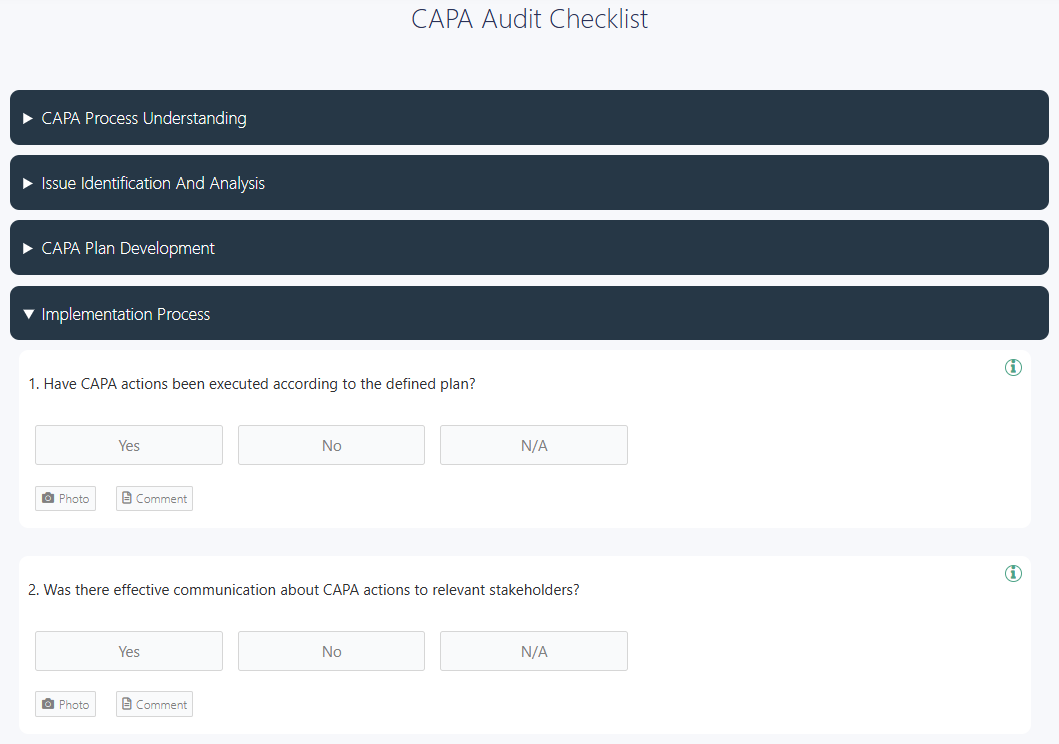
👉 GoAudits offers a free CAPA audit checklist to perform regular CAPA audits.
2. Categorize Findings by Severity and Risk Impact
Evaluate each finding and classify it based on its potential impact on safety, compliance, or operational efficiency. Assign severity levels such as critical, major, or minor. This helps focus resources where they are needed most.
3. Assign Issues to Appropriate Team Members
Allocate each issue to a responsible person or department. Ensure responsibilities are clearly defined, along with due dates and expectations for resolution. Use task management tools to maintain accountability and monitor progress.
4. Initiate Root Cause Analysis
Before implementing solutions, identify the underlying cause of each problem. Apply proven root cause analysis techniques to avoid addressing only symptoms. Document all findings for transparency and future reference.
5. Implement Corrective and Preventive Measures
Corrective actions address the immediate issue, while preventive measures eliminate the chance of recurrence. Both should be specific, measurable, and aligned with industry standards. Clearly record implementation dates and methods.
6. Perform Regular Checks to Verify Effectiveness
Schedule follow-up inspections or process reviews to confirm that corrective and preventive actions are producing the intended results. Use measurable indicators such as defect rates, compliance scores, or incident reports.
7. Record All Actions, Review the CAPA Report, and Share
Maintain a detailed log of every action taken, from discovery to verification. Review the report for completeness and accuracy. Share it with all stakeholders, ensuring they understand the outcomes and lessons learned.
8. Track Trends and Refine Processes Continuously
Analyze CAPA reports over time to identify recurring issues, patterns, or emerging risks. Use these insights to update procedures, training programs, and preventive strategies. This continuous feedback loop strengthens quality management.
Leverage GoAudits for Corrective and Preventive Action Reporting
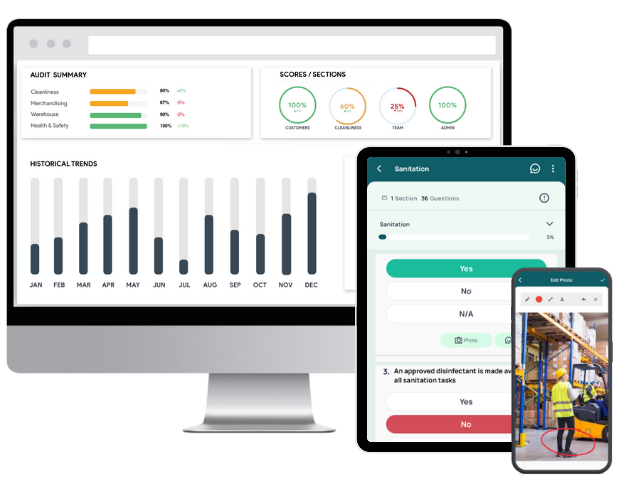
GoAudits is an inspection and auditing tool that helps you catch issues, assign corrective actions, and track them to completion. During inspections, you can flag non-conformities, immediately assign corrective and preventive actions to the right people, set priorities and deadlines, and generate a CAPA audit report automatically. Team members update progress, attach evidence, and request clarifications in the app. Automated alerts and escalation rules keep actions moving, while dashboards show what is overdue, in progress, or completed. GoAudits can help you maintain consistently high standards across all locations:
- Start with ready-to-use industry templates or quickly create custom checklists via a drag-and-drop interface.
- Conduct inspections on any device, even offline, with the ability to attach photos, annotations, signatures, timestamps, and geolocation.
- Automatically generate branded, professional reports at the end of each inspection, complete with visuals, scores, and actionable insights, to demonstrate CAPA compliance anytime
- Automate task assignments, approvals, escalations, and reminders to involve the right people at the right time.
- Use smart dashboards to monitor trends, recurring issues, and performance across locations, teams, and timeframes for better decisions.
Why is a Corrective and Preventive Action Report Important?
A corrective and preventive action report drives quality, efficiency, and continuous improvement within your organization. Here are some reasons why it’s important:
- A CAPA report creates a clear, organized record of problems, investigations, and resolutions. It ensures that each issue is captured with precise details and that corrective and preventive measures are documented for easy reference.
- By centralizing information, a CAPA report enables different teams, such as quality, operations, and management, to access the same data. This eliminates misinterpretation and ensures alignment on actions and responsibilities.
- Documenting issues in a CAPA system triggers faster response times. Clear timelines and responsibilities make it easier to address problems promptly, reducing operational disruptions and potential risks.
- A CAPA report records actions assigned to specific individuals or teams, making it clear who is responsible for implementation. This ensures accountability throughout the problem-resolution process.
- Past CAPA reports help identify recurring issues, understand the effectiveness of past actions, and apply proven solutions to similar problems in the future.
- CAPA documentation enables systematic root cause analysis and verification of implemented solutions. You can track whether the corrective action has truly resolved the issue and whether preventive measures are effective.
- A structured CAPA record makes audits and compliance reporting more efficient. It provides auditors, regulators, and stakeholders with clear evidence of your organization’s commitment to quality and process control.
- CAPA reports demonstrate a proactive approach to problem-solving. It encourages employees to identify, report, and resolve issues, creating a workplace culture that values improvement and accountability.
FAQs
Document corrective actions with clear, measurable steps linked to root cause analysis. Include timelines, responsible parties, and verification methods. Use precise language to eliminate ambiguity. Ensure evidence of implementation is attached. Maintain traceability from problem identification to resolution for audit readiness and regulatory compliance.
Avoid vague problem descriptions, missing root cause analysis, and unverified actions. Do not skip preventive measures or fail to assign accountability. Incomplete documentation and lack of follow-up weaken effectiveness. Ensure every step is supported with evidence and meets regulatory expectations to avoid recurring issues and audit findings.
CAPA integrates by feeding insights into risk management, internal audits, and continuous improvement processes. Findings often trigger updates to SOPs, training, and design controls. Data from CAPA reports support trend analysis in QMS, ensuring corrective measures align with broader quality objectives and regulatory frameworks across operations.
Define problems clearly, perform thorough root cause analysis, and document corrective and preventive actions in detail. Assign responsibilities, set deadlines, and track progress systematically. Verify effectiveness through audits or data reviews. Use CAPA trends for proactive improvements and integrate findings into other quality processes for sustainable compliance.